
Hydroformylation
Encyclopedia
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehyde
s from alkene
s. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen
atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergent
s. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis
of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry
.
The process typically is accomplished by treatment of an alkene
with high pressures (between 10 to 100 atmosphere
s) of carbon monoxide
and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.
, discovered by Otto Roelen
. Subsequent work demonstrated that the ligand tributylphosphine
(PBu3) improved the selectivity of the cobalt-catalysed process. Since the 1970s, most hydroformylation relies on catalysts based on rhodium
. Subsequent research led to the development of water-soluble catalysts that facilitate the separation of the products from the catalyst.
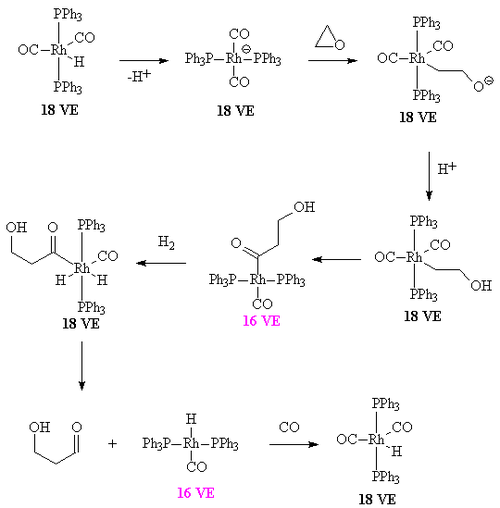
with additional steps. The reaction begins with the generation of coordinatively unsaturated metal hydrido carbonyl complex such as HCo(CO)3 and HRh(CO)(PPh3)2. Such species bind alkenes, and the resulting complex undergoes a migratory insertion
reaction to form an alkyl complex.
can afford two isomer
ic products, butyraldehyde or isobutyraldehyde
:
These isomers result from the differing ways of inserting the alkene into the M-H bond. Of course, both products are not equally desirable. Much research was dedicated to the quest for catalyst that favored the normal isomer.
addition) the resulting alkyl group has a larger steric bulk close to the ligands on the cobalt. If the ligands on the cobalt are bulky (such as tributyl phosphine), then this steric effect is greater. Hence, the mixed carbonyl/phosphine complexes offer a greater selectivity toward the straight chain products.

s that favour the Markovnikov addition to an alkene are less able to direct the hydride to the carbon atom bearing the most hydrogens already. Thus, as a result, as the metal centre becomes more electron-rich, the catalyst becomes more selective for the straight chain compounds.
molecule. One of these hydride
s then takes part in a reductive elimination to form the molecule of the aldehyde
and the complex [HCo(CO)3].
 It is important that the rate of migatory insertion of the carbonyl
It is important that the rate of migatory insertion of the carbonyl
into the carbon
-metal
bond of the alkyl is fast; in systems where the migatory insertion does not occur (such as nickel hydride tristriphenyl phosphite
), the reaction of the hydride with the alkene is reversible. This results in the isomerisation of the alkene, in this way oct-2-ene could be converted into a mixture of both oct-1-ene and oct-2-ene by a beta hydride elimination from the alkyl. In the system below, the rate of insertion of the carbonyl carbon into the C-M bond is likely to be greater than the rate of beta-hydride elimination. If the converse was true then some n-C8H17CHO would have been formed. Hydroformylation of 2-octene: the rhodium
catalyst is coordinated to acac and carbon monoxide
and encapsulated in a molecular self-assembly
process by zinc tetraphenylporphyrin
or Zn-tpp and the pyridine
analogue of triphenylphosphine
. In this process very much like the way enzyme
s work encapsulation of the catalytic site explains the observed regioselectivity
:

s. Using chiral
phosphine ligand
s, the hydroformylation can be tailored to favor one enantiomer
.
and ethylene oxide
to give 2-hydroxyacetaldehyde
and 3-hydroxypropanaldehyde, which can then be hydrogenated to ethylene glycol
and 1,3-propanediol
, respectively. The reactions work best when the solvent is basic (such as pyridine
).
 In the case of dicobalt octacarbonyl
In the case of dicobalt octacarbonyl
or Co2(CO)8 as a catalyst, 2-pentanone can arise from ethylene and CO, in the absence of hydrogen. A proposed intermediate is the ethylene-propionyl species [CH3C(O)Co(CO)3(ethylene)] which undergoes a migratory insertion
to form [CH3COCH2CH2Co(CO)3]. The required hydrogen arises from the water shift reaction. For details, see
 If the water shift reaction is not operative, the reaction affords a polymer containing alternating carbon monoxide and ethylene units. Such aliphatic polyketone
If the water shift reaction is not operative, the reaction affords a polymer containing alternating carbon monoxide and ethylene units. Such aliphatic polyketone
s are more conventionally prepared using palladium
catalysts.
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s from alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
.
The process typically is accomplished by treatment of an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
with high pressures (between 10 to 100 atmosphere
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
s) of carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.
Catalysts
The original catalyst was HCo(CO)4Tetracarbonylhydrocobalt
Cobalt tetracarbonyl hydride is the organometallic compound with the formula HCo4. It is a yellow liquid that forms a colorless vapor and has an intolerable odor. Its main use is as a catalyst in hydroformylation.-Structure and properties:...
, discovered by Otto Roelen
Otto Roelen
Otto Roelen was a German chemist.Roelen studied chemistry and graduated in 1922 from Technische Hochschule Stuttgart. He worked with Franz Fischer and Hans Tropsch at the Kaiser Wilhelm Institute for Coal Research from 1922...
. Subsequent work demonstrated that the ligand tributylphosphine
Tributylphosphine
Tributylphosphine, formula P or PBu, is a tertiary phosphine, most commonly encountered as a ligand in transition metal complexes. It is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with other oxidizing agents, to give the...
(PBu3) improved the selectivity of the cobalt-catalysed process. Since the 1970s, most hydroformylation relies on catalysts based on rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
. Subsequent research led to the development of water-soluble catalysts that facilitate the separation of the products from the catalyst.
Mechanism
The overall mechanism resembles that for homogeneous hydrogenationHydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
with additional steps. The reaction begins with the generation of coordinatively unsaturated metal hydrido carbonyl complex such as HCo(CO)3 and HRh(CO)(PPh3)2. Such species bind alkenes, and the resulting complex undergoes a migratory insertion
Migratory insertion
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the...
reaction to form an alkyl complex.
Selectivity
A key consideration of hydroformylation is the "normal" vs. "iso" selectivity. For example, the hydroformylation of propylenePropylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
can afford two isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
ic products, butyraldehyde or isobutyraldehyde
Isobutyraldehyde
Isobutyraldehyde is the chemical compound with the formula 2CHCHO. It is an aldehyde, isomeric with n-butyraldehyde . Isobutyraldehyde is manufactured, often as a side-product, by the hydroformylation of propene...
:
- H2 + CO + CH3CH=CH2 → CH3CH2CH2CHO ("normal")
- vs.
- H2 + CO + CH3CH=CH2 → (CH3)2CHCHO ("iso")
These isomers result from the differing ways of inserting the alkene into the M-H bond. Of course, both products are not equally desirable. Much research was dedicated to the quest for catalyst that favored the normal isomer.
Steric effects
When the hydrogen is transferred to the carbon bearing the most hydrogen atoms (MarkovnikovMarkovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
addition) the resulting alkyl group has a larger steric bulk close to the ligands on the cobalt. If the ligands on the cobalt are bulky (such as tributyl phosphine), then this steric effect is greater. Hence, the mixed carbonyl/phosphine complexes offer a greater selectivity toward the straight chain products.

Electronic effects
In addition, the more electron-rich the hydride complex is the less proton-like the hydride is. Thus, as a result, the electronic effectElectronic effect
An electronic effect influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry of a...
s that favour the Markovnikov addition to an alkene are less able to direct the hydride to the carbon atom bearing the most hydrogens already. Thus, as a result, as the metal centre becomes more electron-rich, the catalyst becomes more selective for the straight chain compounds.
Acetyl formation
After the alkyl formation a second migatory insertion converts the alkyl into an acetyl ligand (this is when the alkyl carbon forms a bond with the carbon of a carbonyl ligand). The vacant site on the metal is filled by two hydrogens (from the oxidative insertion of a hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
molecule. One of these hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
s then takes part in a reductive elimination to form the molecule of the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
and the complex [HCo(CO)3].

Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
into the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
bond of the alkyl is fast; in systems where the migatory insertion does not occur (such as nickel hydride tristriphenyl phosphite
Triphenyl phosphite
Triphenyl phosphite is the chemical compound with the formula P3. This colourless viscous liquid is the ester of phosphorous acid and phenol. It is used as a ligand in organometallic chemistry...
), the reaction of the hydride with the alkene is reversible. This results in the isomerisation of the alkene, in this way oct-2-ene could be converted into a mixture of both oct-1-ene and oct-2-ene by a beta hydride elimination from the alkyl. In the system below, the rate of insertion of the carbonyl carbon into the C-M bond is likely to be greater than the rate of beta-hydride elimination. If the converse was true then some n-C8H17CHO would have been formed. Hydroformylation of 2-octene: the rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
catalyst is coordinated to acac and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and encapsulated in a molecular self-assembly
Molecular self-assembly
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly, intramolecular self-assembly and intermolecular self-assembly...
process by zinc tetraphenylporphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
or Zn-tpp and the pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
analogue of triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
. In this process very much like the way enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s work encapsulation of the catalytic site explains the observed regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
:

Asymmetric hydroformylation
Hydroformylation of internal alkenes creates new stereocenterStereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s. Using chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
phosphine ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, the hydroformylation can be tailored to favor one enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
.
Other substrates
Cobalt carbonyl and rhodium complexes catalyse the hydroformylation of formaldehydeFormaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
to give 2-hydroxyacetaldehyde
Glycolaldehyde
Glycolaldehyde is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide...
and 3-hydroxypropanaldehyde, which can then be hydrogenated to ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
and 1,3-propanediol
1,3-Propanediol
1,3-Propanediol is the organic compound with the formula CH22. This three-carbon diol is a colorless viscous liquid that is miscible with water.-Products:...
, respectively. The reactions work best when the solvent is basic (such as pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
).

Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
or Co2(CO)8 as a catalyst, 2-pentanone can arise from ethylene and CO, in the absence of hydrogen. A proposed intermediate is the ethylene-propionyl species [CH3C(O)Co(CO)3(ethylene)] which undergoes a migratory insertion
Migratory insertion
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the...
to form [CH3COCH2CH2Co(CO)3]. The required hydrogen arises from the water shift reaction. For details, see

Polyketone
Polyketone Density1240 kg/m3Young's modulus 1500 MPaTensile strength 55 MPaElongation @ break350 %notch test20 kJ/m2Glass temperature15°Cmelting point220°CVicat B205heat transfer coefficient 0.27 W/...
s are more conventionally prepared using palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalysts.
Further reading
- “Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Two Volumes (Paperback) by Boy Cornils (Editor), W. A. Herrmann (Editor). ISBN 3-527-29594-1
- “Rhodium Catalyzed Hydroformylation” P. W. N. M. van Leeuwen, C. Claver Eds.; Springer; (2002). ISBN 1-4020-0421-4
- “Homogeneous Catalysis: Understanding the Art” by Piet W. N. M. van Leeuwen Springer; ISBN 2005. ISBN 1-4020-3176-9
- Imyanitov N.S./ Hydroformylation of Olefins with Rhodium Complexes // Rhodium Express. 1995. No 10 - 11 (May). P. 3 - 62 (Eng). ISSN 0869 - 7876

