
Transesterification
Encyclopedia
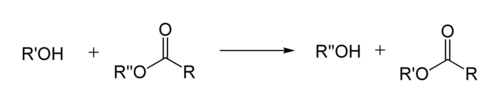
In organic chemistry
, transesterification is the process of exchanging the organic group R″ of an ester
with the organic group R′ of an alcohol
. These reactions are often catalyzed by the addition of an acid
or base
catalyst. The reaction can also be accomplished with the help of enzymes (biocatalysts) particularly lipases (E.C.3.1.1.3).
 Strong acids catalyse the reaction by donating a proton to the carbonyl
Strong acids catalyse the reaction by donating a proton to the carbonyl
group, thus making it a more potent electrophile
, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic
.
s. In this application diesters undergo transesterification with diols to form macromolecules. For example, dimethyl terephthalate
and ethylene glycol
react to form polyethylene terephthalate
and methanol
, which is evaporated to drive the reaction forward.
). It is also used to convert fats (triglycerides) into biodiesel
. This conversion was one of the first uses. Transesterified vegetable oil (biodiesel
) was used to power heavy-duty vehicles in South Africa before World War II
.
It was patented in the U.S.
in the 1950s by Colgate
, though Biolipid
transesterification may have been discovered much earlier. In the 1940s, researchers were looking for a method to more readily produce glycerine, which was used to produce explosives for World War II. Many of the methods used today by producers and homebrewers have their origin in the original 1940s research.
Biolipid
transesterification has also been recently shown by Japanese researchers to be possible using a super-critical methanol methodology, whereby high temperature, high-pressure vessels are used to physically catalyze the Biolipid
/methanol reaction into fatty-acid methyl esters.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, transesterification is the process of exchanging the organic group R″ of an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
with the organic group R′ of an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. These reactions are often catalyzed by the addition of an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
catalyst. The reaction can also be accomplished with the help of enzymes (biocatalysts) particularly lipases (E.C.3.1.1.3).

Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group, thus making it a more potent electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
.
Polyester production
The largest scale application of transesterification is in the synthesis of polyesterPolyester
Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate...
s. In this application diesters undergo transesterification with diols to form macromolecules. For example, dimethyl terephthalate
Dimethyl terephthalate
Dimethyl terephthalate is an organic compound with the formula C6H42. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.-Production:...
and ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
react to form polyethylene terephthalate
Polyethylene terephthalate
Polyethylene terephthalate , commonly abbreviated PET, PETE, or the obsolete PETP or PET-P, is a thermoplastic polymer resin of the polyester family and is used in synthetic fibers; beverage, food and other liquid containers; thermoforming applications; and engineering resins often in combination...
and methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, which is evaporated to drive the reaction forward.
Methanolysis and biodiesel production
The reverse reaction, methanolysis, is also an example of transesterification. This process has been used to recycle polyesters into individual monomers (see plastic recyclingPlastic recycling
Plastic recycling is the process of recovering scrap or waste plastics and reprocessing the material into useful products, sometimes completely different in form from their original state. For instance, this could mean melting down soft drink bottles and then casting them as plastic chairs and tables...
). It is also used to convert fats (triglycerides) into biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
. This conversion was one of the first uses. Transesterified vegetable oil (biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
) was used to power heavy-duty vehicles in South Africa before World War II
World War II
World War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
.
It was patented in the U.S.
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
in the 1950s by Colgate
Colgate-Palmolive
Colgate-Palmolive Company is an American diversified multinational corporation focused on the production, distribution and provision of household, health care and personal products, such as soaps, detergents, and oral hygiene products . Under its "Hill's" brand, it is also a manufacturer of...
, though Biolipid
Biolipid
A biolipid is a lipid from a biological source. The biological sources are:* Vegetable oil* Animal fats...
transesterification may have been discovered much earlier. In the 1940s, researchers were looking for a method to more readily produce glycerine, which was used to produce explosives for World War II. Many of the methods used today by producers and homebrewers have their origin in the original 1940s research.
Biolipid
Biolipid
A biolipid is a lipid from a biological source. The biological sources are:* Vegetable oil* Animal fats...
transesterification has also been recently shown by Japanese researchers to be possible using a super-critical methanol methodology, whereby high temperature, high-pressure vessels are used to physically catalyze the Biolipid
Biolipid
A biolipid is a lipid from a biological source. The biological sources are:* Vegetable oil* Animal fats...
/methanol reaction into fatty-acid methyl esters.
See also
- Biodiesel productionBiodiesel productionBiodiesel production is the process of producing the biofuel, biodiesel, through either transesterification or alcoholysis. It involves reacting vegetable oils or animal fats catalytically with a short-chain aliphatic alcohols ....
- Interesterified fatInteresterified fatInteresterified fat is a type of oil where the fatty acids have been moved from one triglyceride molecule to another. Interesterification does not alter the fatty acids. This is generally done to modify the melting point, slow rancidification and create an oil more suitable for deep frying or...
- Otera's catalyst
- TransalkylationTransalkylationTransalkylation is a chemical reaction of transfer of an alkyl group from one organic compound to another. Zeolite catalysts are often used. The reaction is widely used in the petrochemical industry to manufacture p-xylene, styrene, and other aromatic compounds....

