
Metal nitrosyl
Encyclopedia
Metal nitrosyl complexes are complexes
that contain nitric oxide
, NO, bonded to a transition metal
. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand
.
, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4
illustrate the analogy between NO+ and CO. Similarly, two NO groups are isoelectronic with three CO groups. This trend is illustrated by the isoelectronic pair Fe(CO)2(NO)2 and [Ni(CO)4]. These complexes are isoelectronic and, incidentally, both obey the 18-electron rule
. The formal description of nitric oxide as NO+ does not match certain measureable and calculated properties. In an alternative description, nitric oxide serves as a 3-electron donor, and the metal-nitrogen interaction is a triple bond
.
. A complex with a bent NO ligand is trans-[Co(en)2(NO)Cl]+. Trends in structure and bonding are usually analyzed using the Enemark-Feltham approach. In their framework, the factor that determines the bent vs linear NO ligands in octahedral complexes is the sum of electrons of pi-symmetry. Complexes with "pi-electrons" in excess of 6 tend to have bent NO ligands. Thus, [Co(en)2(NO)Cl]+ with seven electrons of pi-symmetry (six in t2g orbitals and one on NO), adopts a bent NO ligand whereas [Fe(CN)5(NO)]3- with six electrons of pi-symmetry, adopts a linear nitrosyl.
Linear and bent NO ligands can be distinguished using Infrared spectroscopy
. Linear M-N-O groups absorb in the range 1650–1900 cm−1, whereas bent nitrosyls absorb in the range 1525–1690 cm−1. The differing vibrational frequencies reflect the differing N-O bond order
s for linear (triple bond
) and bent NO (double bond
).
. In the compound [Mn3(η5C5H5)3 (μ2-NO)3 (μ3-NO)], three NO groups bridge two metal centres and one NO group bridge to all three.
, which is a sodium salt of the anion [Fe2(NO)4S2]2−. The structure of the anion can be viewed as consisting of two tetrahedra
sharing an edge. Each iron atom is bonded linearly to two NO+ ligands and shares two bridging sulfido ligands with the other iron atom. Roussin's black salt
has a more complex cluster structure. The anion in this species has the formula [Fe4(NO)7S3]-. It has C3v symmetry. It consists of a tetrahedron of iron atoms with sufide ions on three faces of the tetrahedron. Three iron atoms are bonded to two nitrosyl groups. The iron atom on the threefold symmetry axis has a single nitrosyl group which also lies on that axis.
In such approaches one must guard against the tendency of nitric oxide to be oxidized by air. Replacement of ligands by the nitrosyl cation may be accomplished using nitrosyl tetrafluoroborate, [NO][BF4]. Other indirect methods are indirect with the NO group deriving from some other species, often accompanied by oxidation and reduction reactions. A classic example is provided by the brown ring test
in which the nitrate ion is the source of a nitric oxide ligand. Nitrosyl chloride is also useful, being applicable to [Mo(NO)2Cl2]n.
This equilibrium serves to confirm that the linear nitrosyl ligand is, formally, NO+, with nitrogen in the oxidation state +3
Since nitrogen is more electronegative than carbon, metal-nitrosyl complexes tend to be more electrophilic than related metal carbonyl complexes. Nucleophiles often add to the nitrogen. The nitrogen atom in bent metal nitrosyls is basic, thus can be oxidized, alkylated, and protonated, e.g.:2(CO)ClOsNO + HCl → (Ph3P)2(CO)ClOsN(H)O
In rare cases, NO is cleaved by metal centers:
function of NO is effected via its complexation to haeme proteins, where it binds in the bent geometry.
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
that contain nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
, NO, bonded to a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
.
Bonding and structure
Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4
Nickel carbonyl
Nickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
illustrate the analogy between NO+ and CO. Similarly, two NO groups are isoelectronic with three CO groups. This trend is illustrated by the isoelectronic pair Fe(CO)2(NO)2 and [Ni(CO)4]. These complexes are isoelectronic and, incidentally, both obey the 18-electron rule
18-Electron rule
The 18-electron rule is a rule of thumb used primarily for predicting formulas for stable metal complexes. The rule rests on the fact that valence shells of a transition metal consists of nine valence orbitals, which collectively can accommodate 18 electrons either as nonbinding electron pairs or...
. The formal description of nitric oxide as NO+ does not match certain measureable and calculated properties. In an alternative description, nitric oxide serves as a 3-electron donor, and the metal-nitrogen interaction is a triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
.

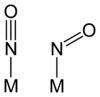
Linear vs bent nitrosyl ligands
The M-N-O unit in nitrosyl complexes is usually linear, or no more than 15° from linear. In some complexes, however, especially when back-bonding is less important, the M-N-O angle can strongly deviate from 180°. In such cases, the NO ligand is sometimes described as the anion, NO-. Prototypes for such compounds are the organic nitroso compounds, such as nitrosobenzeneNitrosobenzene
Nitrosobenzene is the organic compound with the formula C6H5NO. The compound can be viewed as hybrid of singlet O2 and azobenzene. This diamagnetic species exists in equilibrium with its dimer.-Preparation:...
. A complex with a bent NO ligand is trans-[Co(en)2(NO)Cl]+. Trends in structure and bonding are usually analyzed using the Enemark-Feltham approach. In their framework, the factor that determines the bent vs linear NO ligands in octahedral complexes is the sum of electrons of pi-symmetry. Complexes with "pi-electrons" in excess of 6 tend to have bent NO ligands. Thus, [Co(en)2(NO)Cl]+ with seven electrons of pi-symmetry (six in t2g orbitals and one on NO), adopts a bent NO ligand whereas [Fe(CN)5(NO)]3- with six electrons of pi-symmetry, adopts a linear nitrosyl.
Linear and bent NO ligands can be distinguished using Infrared spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
. Linear M-N-O groups absorb in the range 1650–1900 cm−1, whereas bent nitrosyls absorb in the range 1525–1690 cm−1. The differing vibrational frequencies reflect the differing N-O bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
s for linear (triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
) and bent NO (double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
).
Bridging nitrosyl ligands
Nitric oxide can also serve as a bridging ligandBridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are...
. In the compound [Mn3(η5C5H5)3 (μ2-NO)3 (μ3-NO)], three NO groups bridge two metal centres and one NO group bridge to all three.
Homoleptic nitrosyl complexes
Metal complexes containing only nitrosyl ligands are called isoleptic nitrosyls. They are rare, the premier member being Cr(NO)4. Even trinitrosyl complexes are uncommon, whereas polycarbonyl complexes are routine.Roussin Red and Black Salts
One of the earliest examples of a nitrosyl complex to be synthesized is Roussin's Red SaltRoussin's Red Salt
Roussin’s Red Salt is the inorganic compound with the formula K2[Fe2S24]. This metal nitrosyl was first described in 1858, making it the first synthetic iron-sulfur cluster.-Structure and bonding:...
, which is a sodium salt of the anion [Fe2(NO)4S2]2−. The structure of the anion can be viewed as consisting of two tetrahedra
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
sharing an edge. Each iron atom is bonded linearly to two NO+ ligands and shares two bridging sulfido ligands with the other iron atom. Roussin's black salt
Roussin's black salt
Roussin's Black Salt is a chemical compound with the formula NaFe4S37. It is classified as metal nitrosyl compound. It consists of the sodium salt of the [Fe4S37]− anion. The geometry of the anion cluster is that of an incomplete cubane...
has a more complex cluster structure. The anion in this species has the formula [Fe4(NO)7S3]-. It has C3v symmetry. It consists of a tetrahedron of iron atoms with sufide ions on three faces of the tetrahedron. Three iron atoms are bonded to two nitrosyl groups. The iron atom on the threefold symmetry axis has a single nitrosyl group which also lies on that axis.
Preparation
Nitrosyl complexes can be prepared by many routes. Direct formation from nitric oxide is common. The nitrosylation of cobalt carbonyl is illustrative:- Co2(CO)8 + 2 NO → 2 CoNO(CO)3 + 2 CO
In such approaches one must guard against the tendency of nitric oxide to be oxidized by air. Replacement of ligands by the nitrosyl cation may be accomplished using nitrosyl tetrafluoroborate, [NO][BF4]. Other indirect methods are indirect with the NO group deriving from some other species, often accompanied by oxidation and reduction reactions. A classic example is provided by the brown ring test
Nitrate test
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble...
in which the nitrate ion is the source of a nitric oxide ligand. Nitrosyl chloride is also useful, being applicable to [Mo(NO)2Cl2]n.
Reactions
An important reaction is the acid/base equilibrium:- [LnMNO]2+ + 2OH- LnMNO2 + H2O
This equilibrium serves to confirm that the linear nitrosyl ligand is, formally, NO+, with nitrogen in the oxidation state +3
- NO+ + 2 OH- NO2- + H2O
Since nitrogen is more electronegative than carbon, metal-nitrosyl complexes tend to be more electrophilic than related metal carbonyl complexes. Nucleophiles often add to the nitrogen. The nitrogen atom in bent metal nitrosyls is basic, thus can be oxidized, alkylated, and protonated, e.g.:2(CO)ClOsNO + HCl → (Ph3P)2(CO)ClOsN(H)O
In rare cases, NO is cleaved by metal centers:
- CpCyclopentadienylIn organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
2NbMe2 + NO → Cp2(Me)Nb(O)NMe - 2 Cp2(Me)Nb(O)NMe → 2 Cp2Nb(O)Me + ½MeN=NMe
Applications
Metal-catalyzed reactions of NO are not commonly synthetically useful. Nitric oxide is however an important signalling molecule in nature and this fact is the basis of the most important applications of metal nitrosyls. The nitroprusside anion,[Fe(CN)5NO]2−, a mixed nitrosyl cyano complex, has pharmaceutical applications as a slow release agent for NO. The signallingCell signaling
Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. The ability of cells to perceive and correctly respond to their microenvironment is the basis of development, tissue repair, and immunity as well as normal tissue...
function of NO is effected via its complexation to haeme proteins, where it binds in the bent geometry.

