
Isocyanide
Encyclopedia
An isocyanide is an organic compound
with the functional group
-N≡C. It is the isomer
of the related cyanide
(-C≡N), hence the prefix iso.
The organic fragment is connected to the isocyanide group via the nitrogen
atom, not via the carbon
.
in most cases the suffix
"nitrile" or "carbonitrile" is used for organic cyanide
s (R-C≡N),
names for isocyanides have the prefix
"isocyano". IUPAC names become isocyanomethane, isocyanoethane, isocyanopropane, et cetera.
The use of the prefix "isonitrile" has a contradiction in the nomenclature. For example, ethyl nitrile ( CH3CN) and ethyl isonitrile (C2H5NC) are not isomers, as the prefix "iso" suggests. In contrast, ethyl cyanide (C2H5CN) and ethyl isocyanide (C2H5NC) are isomers.
The sometimes used term "carbylamine" conflicts with systematic nomenclature. An amine
always has three single bonds,
whereas an isocyanide has only one single and one multiple bond.
, one with a triple bond
between the nitrogen and the carbon and one with a double bond
between. Surprisingly, the first one, with a positively charged nitrogen and a negatively charged carbon is the most important one and describes most closely the resonance hybrid. Isocyanides are isoelectronic with CO
. Thus, the C=N-CR3 (R = hydrogen or an organic group) is linear. They are susceptible to polymerization
.
Ivar Karl Ugi
states that "The development of the chemistry of isocyanides has probably suffered ... through the characteristic odor of volatile isonitriles, which has been described by Hofmann and Gautier as ‘highly specific, almost overpowering’, ‘horrible’, and ‘extremely distressing’. It is true that many potential workers in this field have been turned away by the odour.” Isocyanides have been investigated as potential non-lethal weapons.
Some isocyanides convey less offensive odours such as malt, natural rubber, creosote, mild cherry or old wood.
Non-volatile derivatives such as tosylmethyl isocyanide do not have objectionable odors.
for mammals".
Toxicological studies in the 1960's at Farbenfabriken Bayer AG showed that "oral and subcutaneous doses of 500-5000 mg/kg can be tolerated by mice".
The electronic symmetry about the isocyanide 14N nucleus results in a slow quadrupolar relaxation
so that 13C-14N nuclear spin coupling can be observed, with coupling constants of ca. 5 Hz for the isocyanide 13C nucleus and 5-14 Hz for the 13C nucleus which the isocyanide group is attached to.
isocyanide was prepared in 1859 by the chemist Lieke from the reaction of
allyl
iodide and silver cyanide
. Normally the alkylation
of an alkali metal cyanide gives a nitrile, but the silver ion protects the carbon end of the cyanide. Commonly, isocyanides are synthesized by the reaction of primary amine
s with dichlorocarbene
or by dehydration
of a formamide
with phosphorus oxychloride.
The Hofmann isocyanide synthesis is a chemical test
for primary amine
s based on their reaction with potassium hydroxide
and chloroform
as dichlorocarbene
precursors to foul smelling isocyanides.
Another route to isocyanides is by reaction of organolithium compounds with oxazole
s and benzoxazole
s:
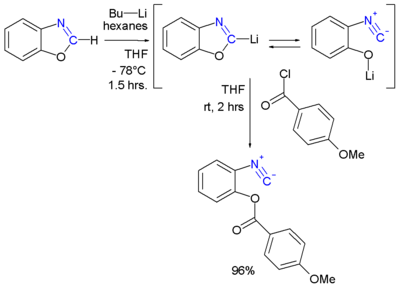 The benzoxazole
The benzoxazole
gets deprotonated at the 2-position by n-butyllithium
. The lithium compound is in chemical equilibrium
with the 2-isocyanophenolate which can be captured by an electrophile
such as an acid chloride. Being an ester
the formed isocyanate in the example above behaves uncharacteristically with reportedly a mild cherry
smell.
Another synthetic route towards an isocyanide is 1) condensation of an amine with formic acid, yielding a formamide, and 2) dehydrating this formamide. Phosgene
(or a synthon or precursor such as diphosgene
) can be used in combination with formamide to yield isocyanides.
Isocyanides are reactants in many multicomponent reactions of interest in organic synthesis
, two of which are: the Ugi reaction
and the Passerini reaction
.
Isocyanides participate in cycloaddition
reactions, such as the [4+1] cycloaddition with tetrazines. Depending on the degree of substitution of the isocyanide, this reaction converts isocyanides into carbonyl
s or gives stable cycloadducts.
later was used as the antibiotic
. Since then numerous other isocyanides have been isolated. Most of the marine isocyanides are terpenes, while some of the terrestrial isocyanides originate from α-aminoacids.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
-N≡C. It is the isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
of the related cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
(-C≡N), hence the prefix iso.
The organic fragment is connected to the isocyanide group via the nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atom, not via the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
.
Nomenclature
Whereas in IUPAC nomenclatureIUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
in most cases the suffix
Suffix
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns or adjectives, and verb endings, which form the conjugation of verbs...
"nitrile" or "carbonitrile" is used for organic cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
s (R-C≡N),
names for isocyanides have the prefix
Prefix
A prefix is an affix which is placed before the root of a word. Particularly in the study of languages,a prefix is also called a preformative, because it alters the form of the words to which it is affixed.Examples of prefixes:...
"isocyano". IUPAC names become isocyanomethane, isocyanoethane, isocyanopropane, et cetera.
The use of the prefix "isonitrile" has a contradiction in the nomenclature. For example, ethyl nitrile ( CH3CN) and ethyl isonitrile (C2H5NC) are not isomers, as the prefix "iso" suggests. In contrast, ethyl cyanide (C2H5CN) and ethyl isocyanide (C2H5NC) are isomers.
The sometimes used term "carbylamine" conflicts with systematic nomenclature. An amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
always has three single bonds,
whereas an isocyanide has only one single and one multiple bond.
Physical properties
Isocyanides are described by two resonance structuresResonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
, one with a triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
between the nitrogen and the carbon and one with a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
between. Surprisingly, the first one, with a positively charged nitrogen and a negatively charged carbon is the most important one and describes most closely the resonance hybrid. Isocyanides are isoelectronic with CO
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. Thus, the C=N-CR3 (R = hydrogen or an organic group) is linear. They are susceptible to polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
.
Odour of isocyanides
Their disagreeable odour is legendary. To quote from Lieke, "Es besitzt einen penetranten, höchst unangenehmen Geruch; das Oeffnen eines Gefässes mit Cyanallyl reicht hin, die Luft eines Zimmers mehrere Tage lang zu verpesten, ..." (It has a penetrating, extremely unpleasant odour; the opening of a flask of allyl [iso]cyanide is enough to foul up the air in a room for several days). Note that in Lieke's day, the difference between isocyanide and nitrile was not fully appreciated.Ivar Karl Ugi
Ivar Karl Ugi
Ivar Karl Ugi was a German chemist who made major contributions to organic chemistry. He is known for the research on multicomponent reactions, yielding the Ugi reaction.-Biography:...
states that "The development of the chemistry of isocyanides has probably suffered ... through the characteristic odor of volatile isonitriles, which has been described by Hofmann and Gautier as ‘highly specific, almost overpowering’, ‘horrible’, and ‘extremely distressing’. It is true that many potential workers in this field have been turned away by the odour.” Isocyanides have been investigated as potential non-lethal weapons.
Some isocyanides convey less offensive odours such as malt, natural rubber, creosote, mild cherry or old wood.
Non-volatile derivatives such as tosylmethyl isocyanide do not have objectionable odors.
Toxicity
While some isocyanides (e.g. cyclohexyl isocyanide) are toxic, others "exhibit no appreciable toxicityfor mammals".
Toxicological studies in the 1960's at Farbenfabriken Bayer AG showed that "oral and subcutaneous doses of 500-5000 mg/kg can be tolerated by mice".
Spectroscopy
IR absorption: 2165-2110 cm-1The electronic symmetry about the isocyanide 14N nucleus results in a slow quadrupolar relaxation
Relaxation (NMR)
In nuclear magnetic resonance spectroscopy and magnetic resonance imaging the term relaxation describes several processes by which nuclear magnetization prepared in a non-equilibrium state return to the equilibrium distribution. In other words, relaxation describes how fast spins "forget" the...
so that 13C-14N nuclear spin coupling can be observed, with coupling constants of ca. 5 Hz for the isocyanide 13C nucleus and 5-14 Hz for the 13C nucleus which the isocyanide group is attached to.
Synthesis of isocyanides
The first isocyanide, allylAllyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
isocyanide was prepared in 1859 by the chemist Lieke from the reaction of
allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
iodide and silver cyanide
Silver cyanide
Silver cyanide is the chemical compound with the formula AgCN. This white solid forms upon treatment of solutions containing Ag+ with cyanide. This precipitation step is used in some schemes to recover silver from solution...
. Normally the alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of an alkali metal cyanide gives a nitrile, but the silver ion protects the carbon end of the cyanide. Commonly, isocyanides are synthesized by the reaction of primary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s with dichlorocarbene
Dichlorocarbene
Dichlorocarbene is a carbene commonly encountered in organic chemistry. This reactive intermediate with chemical formula CCl2 is easily available by reaction of chloroform and a base such as potassium t-butoxide or sodium hydroxide dissolved in water...
or by dehydration
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
of a formamide
Formamide
Formamide, also known as methanamide, is an amide derived from formic acid. It is a clear liquid which is miscible with water and has an ammonia-like odor. It is used primarily for manufacturing sulfa drugs and synthesizing vitamins and as a softener for paper and fiber...
with phosphorus oxychloride.
- RNH2 + :CCl2 + 2 NaOH → RNC + 2 NaCl + 2 H2O
- RNHC(O)H + POCl3 → RNC + "PO2Cl" + 2 HCl
The Hofmann isocyanide synthesis is a chemical test
Chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to prove the existence of, or to quantify, a chemical compound or chemical group with the aid of a specific reagent...
for primary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s based on their reaction with potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
and chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
as dichlorocarbene
Dichlorocarbene
Dichlorocarbene is a carbene commonly encountered in organic chemistry. This reactive intermediate with chemical formula CCl2 is easily available by reaction of chloroform and a base such as potassium t-butoxide or sodium hydroxide dissolved in water...
precursors to foul smelling isocyanides.
Another route to isocyanides is by reaction of organolithium compounds with oxazole
Oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles...
s and benzoxazole
Benzoxazole
Benzoxazole is an aromatic organic compound with a molecular formula C7H5NO, a benzene-fused oxazole ring structure, and an odor similar to pyridine. Benzoxazole is used primarily in industry and research, and has no household use....
s:

Benzoxazole
Benzoxazole is an aromatic organic compound with a molecular formula C7H5NO, a benzene-fused oxazole ring structure, and an odor similar to pyridine. Benzoxazole is used primarily in industry and research, and has no household use....
gets deprotonated at the 2-position by n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
. The lithium compound is in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with the 2-isocyanophenolate which can be captured by an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
such as an acid chloride. Being an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
the formed isocyanate in the example above behaves uncharacteristically with reportedly a mild cherry
Cherry
The cherry is the fruit of many plants of the genus Prunus, and is a fleshy stone fruit. The cherry fruits of commerce are usually obtained from a limited number of species, including especially cultivars of the wild cherry, Prunus avium....
smell.
Another synthetic route towards an isocyanide is 1) condensation of an amine with formic acid, yielding a formamide, and 2) dehydrating this formamide. Phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
(or a synthon or precursor such as diphosgene
Diphosgene
Diphosgene is a chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds...
) can be used in combination with formamide to yield isocyanides.
Reactions
Isocyanides are stable to strong base (they are often made under strongly basic conditions), but they are sensitive to acid. In the presence of aqueous acid, isocyanides hydrolyse to the corresponding formamides. However, some isocyanides can polymerize in the presence of acids. Acid-hydrolysis is a convenient method for removing the obnoxiously odiferous isocyanides.Isocyanides are reactants in many multicomponent reactions of interest in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, two of which are: the Ugi reaction
Ugi reaction
The Ugi reaction is a multi-component reaction in organic chemistry involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide.The reaction is named after Ivar Karl Ugi, who first published this reaction in 1959....
and the Passerini reaction
Passerini reaction
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde , and a carboxylic acid to form a α-acyloxy amide. This organic reaction was discovered by Mario Passerini in 1921 in Florence, Italy...
.
Isocyanides participate in cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
reactions, such as the [4+1] cycloaddition with tetrazines. Depending on the degree of substitution of the isocyanide, this reaction converts isocyanides into carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s or gives stable cycloadducts.
Naturally occurring isocyanides
Several organic molecules extracted from living organisms contain the isocyanide functionality. The first was discovered in 1957 in an extract of the mold Penicillium notatum Westling. The compound xanthocillinXanthocillin
Xantocillin , also known as xanthocillin X or ophthocillin, was the first reported natural product found to contain the isocyanide functional group. It was first isolated from Penicillium notatum by Westling in 1950 and subsequently from several other sources....
later was used as the antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
. Since then numerous other isocyanides have been isolated. Most of the marine isocyanides are terpenes, while some of the terrestrial isocyanides originate from α-aminoacids.

