
Gas-liquid chromatography
Encyclopedia
Gas chromatography is a common type of chromatography
used in analytical chemistry
for separating
and analysing compounds that can be vaporized without decomposition
. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
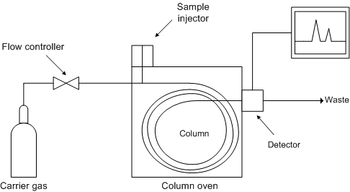
In gas chromatography, the mobile phase (or "moving phase") is a carrier gas
, usually an inert
gas such as helium
or an unreactive gas such as nitrogen
. The stationary phase is a microscopic layer of liquid
or polymer
on an inert solid
support, inside a piece of glass
or metal
tubing called a column (an homage to the fractionating column
used in distillation). The instrument used to perform gas chromatography is called a gas chromatograph (or "aerograph", "gas separator").
The gaseous compounds being analyzed interact with the walls of the column, which is coated with different stationary phases. This causes each compound to elute
at a different time, known as the retention time of the compound. The comparison of retention times is what gives GC its analytical usefulness.
Gas chromatography is in principle similar to column chromatography
(as well as other forms of chromatography, such as HPLC
, TLC
), but has several notable differences. Firstly, the process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas mobile phase, whereas in column chromatography the stationary phase is a solid and the mobile phase is a liquid. (Hence the full name of the procedure is "Gas–liquid chromatography", referring to the mobile and stationary phases, respectively.) Secondly, the column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled, whereas column chromatography (typically) has no such temperature control. Thirdly, the concentration of a compound in the gas phase is solely a function
of the vapor pressure
of the gas.
Gas chromatography is also similar to fractional distillation
, since both processes separate the components of a mixture primarily based on boiling point
(or vapor pressure) differences. However, fractional distillation is typically used to separate components of a mixture on a large scale, whereas GC can be used on a much smaller scale (i.e. microscale).
Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently found in scientific literature. Strictly speaking, GLPC is the most correct terminology, and is thus preferred by many authors.
dates to 1903 in the work of the Russian scientist, Mikhail Semenovich Tswett. German
graduate student Fritz Prior developed solid state gas chromatography in 1947. Archer John Porter Martin
, who was awarded the Nobel Prize
for his work in developing liquid–liquid (1941) and paper (1944) chromatography, laid the foundation for the development of gas chromatography and he later produced liquid-gas chromatography (1950). Erika Cremer
laid the groundwork, and oversaw much of Prior's work.
In a GC analysis, a known volume of gaseous or liquid analyte
is injected into the "entrance" (head) of the column, usually using a microsyringe
(or, solid phase microextraction fibers, or a gas source switching system). As the carrier gas sweeps the analyte molecules through the column, this motion is inhibited by the adsorption
of the analyte molecule
s either onto the column walls or onto packing materials in the column. The rate at which the molecules progress along the column depends on the strength of adsorption
, which in turn depends on the type of molecule and on the stationary phase materials. Since each type of molecule has a different rate of progression, the various components of the analyte mixture are separated as they progress along the column and reach the end of the column at different times (retention time). A detector is used to monitor the outlet stream from the column; thus, the time at which each component reaches the outlet and the amount of that component can be determined. Generally, substances are identified (qualitatively) by the order in which they emerge (elute) from the column and by the retention time of the analyte in the column.
the means to introduce a sample automatically into the inlets. Manual insertion of the sample is possible but is no longer common. Automatic insertion provides better reproducibility and time-optimization.
Different kinds of autosamplers exist. Autosamplers can be classified in relation to sample capacity (auto-injectors vs. autosamplers, where auto-injectors can work a small number of samples), to robotic technologies (XYZ robot vs. rotating robot – the most common), or to analysis:
Traditionally autosampler manufacturers are different from GC manufacturers and currently no GC manufacturer offers a complete range of autosamplers. Historically, the countries most active in autosampler technology development are the United States, Italy, Switzerland, and the United Kingdom.
Common inlet types are:
The choice of carrier gas (mobile phase) is important, with hydrogen being the most efficient and providing the best separation. However, helium has a larger range of flowrates that are comparable to hydrogen in efficiency, with the added advantage that helium is non-flammable, and works with a greater number of detectors. Therefore, helium is the most common carrier gas used.
Detectoron are the flame ionization detector
(FID) and the thermal conductivity detector
(TCD). Both are sensitive to a wide range of components, and both work over a wide range of concentrations. While TCDs are essentially universal and can be used to detect any component other than the carrier gas (as long as their thermal conductivities are different from that of the carrier gas, at detector temperature), FIDs are sensitive primarily to hydrocarbons, and are more sensitive to them than TCD. However, an FID cannot detect water. Both detectors are also quite robust. Since TCD is non-destructive, it can be operated in-series before an FID (destructive), thus providing complementary detection of the same analytes.
Other detectors are sensitive only to specific types of substances, or work well only in narrower ranges of concentrations. They include:
Some gas chromatographs are connected to a mass spectrometer which acts as the detector. The combination is known as GC-MS. Some GC-MS are connected to an NMR spectrometer which acts as a backup detector. This combination is known as GC-MS-NMR. Some GC-MS-NMR are connected to an infrared spectrophotometer
which acts as a backup detector. This combination is known as GC-MS-NMR-IR. It must, however, be stressed this is very rare as most analyses needed can be concluded via purely GC-MS.
Conditions which can be varied to accommodate a required analysis include inlet temperature, detector temperature, column temperature and temperature program, carrier gas and carrier gas flow rates, the column's stationary phase, diameter and length, inlet type and flow rates, sample size and injection technique. Depending on the detector(s) (see below) installed on the GC, there may be a number of detector conditions that can also be varied. Some GCs also include valves which can change the route of sample and carrier flow. The timing of the opening and closing of these valves can be important to method development.

This image above shows the interior of a GeoStrata Technologies Eclipse Gas Chromatograph that runs continuously in three minute cycles. Two valves are used to switch the test gas into the sample loop. After filling the sample loop with test gas, the valves are switched again applying carrier gas pressure to the sample loop and forcing the sample through the Column for separation.
Carrier gas selection and flow rates
Typical carrier gases include helium
, nitrogen
, argon
, hydrogen
and air. Which gas to use is usually determined by the detector being used, for example, a DID
requires helium as the carrier gas. When analyzing gas samples, however, the carrier is sometimes selected based on the sample's matrix, for example, when analyzing a mixture in argon, an argon carrier is preferred, because the argon in the sample does not show up on the chromatogram. Safety and availability can also influence carrier selection, for example, hydrogen is flammable, and high-purity helium can be difficult to obtain in some areas of the world. (See: Helium—occurrence and production.) As a result of helium becoming more scarce, hydrogen is often being substituted for helium as a carrier gas in several applications.
The purity of the carrier gas is also frequently determined by the detector, though the level of sensitivity needed can also play a significant role. Typically, purities of 99.995% or higher are used. The most common purity grades required by modern instruments for the majority of sensitivities are 5.0 grades, or 99.999% pure meaning that there is a total of 10ppm of impurities in the carrier gas that could affect the results. The highest purity grades in common use are 6.0 grades, but the need for detection at very low levels in some forensic and environmental applications has driven the need for carrier gases at 7.0 grade purity and these are now commercially available. Trade names for typical purities include "Zero Grade," "Ultra-High Purity (UHP) Grade," "4.5 Grade" and "5.0 Grade."
The carrier gas linear velocity affects the analysis in the same way that temperature does (see above). The higher the linear velocity the faster the analysis, but the lower the separation between analytes. Selecting the linear velocity is therefore the same compromise between the level of separation and length of analysis as selecting the column temperature. The linear velocity will be implemented by means of the carrier gas flow rate, with regards to the inner diameter of the column.
With GCs made before the 1990s, carrier flow rate was controlled indirectly by controlling the carrier inlet pressure, or "column head pressure." The actual flow rate was measured at the outlet of the column or the detector with an electronic flow meter, or a bubble flow meter, and could be an involved, time consuming, and frustrating process. The pressure setting was not able to be varied during the run, and thus the flow was essentially constant during the analysis. The relation between flow rate and inlet pressure is calculated with Poiseuille's equation for compressible fluids.
Many modern GCs, however, electronically measure the flow rate, and electronically control the carrier gas pressure to set the flow rate. Consequently, carrier pressures and flow rates can be adjusted during the run, creating pressure/flow programs similar to temperature programs.
Stationary compound selection
The polarity
of the solute is crucial for the choice of stationary compound, which in an optimal case would have a similar polarity than the solute. Common stationary phases in open tubular columns are cyanopropylphenyl dimethyl polysiloxane, carbowax polyethyleneglycol, biscyanopropyl cyanopropylphenyl polysiloxane and diphenyl dimethyl polysiloxane. For packed columns more options are available.
Inlet types and flow rates
The choice of inlet type and injection technique depends on if the sample is in liquid, gas, adsorbed, or solid form, and on whether a solvent matrix is present that has to be vaporized. Dissolved samples can be introduced directly onto the column via a COC injector, if the conditions are well known; if a solvent matrix has to be vaporized and partially removed, a S/SL injector is used (most common injection technique); gaseous samples (e.g., air cylinders) are usually injected using a gas switching valve system; adsorbed samples (e.g., on adsorbent tubes) are introduced using either an external (on-line or off-line) desorption apparatus such as a purge-and-trap system, or are desorbed in the S/SL injector (SPME applications).
 The real chromatographic analysis starts with the introduction of the sample onto the column. The development of capillary gas chromatography resulted in many practical problems with the injection technique. The technique of on-column injection, often used with packed columns, is usually not possible with capillary columns. The injection system in the capillary gas chromatograph should fulfil the following two requirements:
The real chromatographic analysis starts with the introduction of the sample onto the column. The development of capillary gas chromatography resulted in many practical problems with the injection technique. The technique of on-column injection, often used with packed columns, is usually not possible with capillary columns. The injection system in the capillary gas chromatograph should fulfil the following two requirements:
Some general requirements which a good injection technique should fulfill are:
Select dimensions of column for corresponding samples and GC System[Limit Temp][Dec-2009]'
Column selection
The choice of column depends on the sample and the active measured. The main chemical attribute regarded when choosing a column is the polarity
of the mixture, but functional groups can play a large part in column selection. The polarity of the sample must closely match the polarity of the column stationary phase to increase resolution and separation while reducing run time. The separation and run time also depends on the film thickness (of the stationary phase), the column diameter and the column length.
Column temperature and temperature program
 The column(s) in a GC are contained in an oven, the temperature of which is precisely controlled electronically. (When discussing the "temperature of the column," an analyst is technically referring to the temperature of the column oven. The distinction, however, is not important and will not subsequently be made in this article.)
The column(s) in a GC are contained in an oven, the temperature of which is precisely controlled electronically. (When discussing the "temperature of the column," an analyst is technically referring to the temperature of the column oven. The distinction, however, is not important and will not subsequently be made in this article.)
The rate at which a sample passes through the column is directly proportional to the temperature of the column. The higher the column temperature, the faster the sample moves through the column. However, the faster a sample moves through the column, the less it interacts with the stationary phase, and the less the analytes are separated.
In general, the column temperature is selected to compromise between the length of the analysis and the level of separation.
A method which holds the column at the same temperature for the entire analysis is called "isothermal." Most methods, however, increase the column temperature during the analysis, the initial temperature, rate of temperature increase (the temperature "ramp") and final temperature is called the "temperature program."
A temperature program allows analytes that elute early in the analysis to separate adequately, while shortening the time it takes for late-eluting analytes to pass through the column.
Generally chromatographic data is presented as a graph of detector response (y-axis) against retention time (x-axis), which is called a chromatogram. This provides a spectrum of peaks for a sample representing the analyte
s present in a sample eluting from the column at different times. Retention time can be used to identify analytes if the method conditions are constant. Also, the pattern of peaks will be constant for a sample under constant conditions and can identify complex mixtures of analytes. In most modern applications however the GC is connected to a mass spectrometer
or similar detector that is capable of identifying the analytes represented by the peaks.
Quantitative analysis:
The area under a peak is proportional to the amount of analyte present in the chromatogram. By calculating the area of the peak using the mathematical function of integration, the concentration of an analyte in the original sample can be determined. Concentration can be calculated using a calibration curve
created by finding the response for a series of concentrations of analyte, or by determining the relative response factor of an analyte. The relative response factor is the expected ratio of an analyte to an internal standard
(or external standard) and is calculated by finding the response of a known amount of analyte and a constant amount of internal standard (a chemical added to the sample at a constant concentration, with a distinct retention time to the analyte).
In most modern GC-MS systems, computer software
is used to draw and integrate peaks, and match MS
spectra to library spectra.
-free; they should not contain ion
s. Very minute amounts of a substance can be measured, but it is often required that the sample must be measured in comparison to a sample containing the pure, suspected substance known as a reference standard
.
Various temperature programs can be used to make the readings more meaningful; for example to differentiate between substances that behave similarly during the GC process.
Professionals working with GC analyze the content of a chemical product, for example in assuring the quality of products in the chemical industry; or measuring toxic substances in soil, air or water. GC is very accurate if used properly and can measure picomoles of a substance in a 1 ml liquid sample, or parts-per-billion concentrations in gaseous samples.
In practical courses at colleges, students sometimes get acquainted to the GC by studying the contents of Lavender
oil or measuring the ethylene
that is secreted by Nicotiana benthamiana
plants after artificially injuring their leaves. These GC analyses hydrocarbons (C2-C40+). In a typical experiment, a packed column is used to separate the light gases, which are then detected with a TCD
. The hydrocarbon
s are separated using a capillary column and detected with an FID
. A complication with light gas analyses that include H2 is that He, which is the most common and most sensitive inert carrier (sensitivity is proportional to molecular mass) has an almost identical thermal conductivity to hydrogen (it is the difference in thermal conductivity between two separate filaments in a Wheatstone Bridge type arrangement that shows when a component has been eluted). For this reason, dual TCD instruments are used with a separate channel for hydrogen that uses nitrogen as a carrier are common. Argon is often used when analysing gas phase chemistry reactions such as F-T synthesis so that a single carrier gas can be used rather than 2 separate ones. The sensitivity is less but this is a tradeoff for simplicity in the gas supply.
In the U.S. TV show CSI
, for example, GCs are used to rapidly identify unknown samples. "This is gasoline
bought at a Chevron
station in the past two weeks," the analyst will say fifteen minutes after receiving the sample.
In fact, a typical GC analysis takes much more time; sometimes a single sample must be run more than an hour according to the chosen program; and even more time is needed to "heat out" the column so it is free from the first sample and can be used for the next. Equally, several runs are needed to confirm the results of a study – a GC analysis of a single sample may simply yield a result per chance (see statistical significance
).
Also, GC does not positively identify most samples; and not all substances in a sample will necessarily be detected. All a GC truly tells you is at which relative time a component eluted from the column and that the detector was sensitive to it. To make results meaningful, analysts need to know which components at which concentrations are to be expected; and even then a small amount of a substance can hide itself behind a substance having both a higher concentration and the same relative elution time. Last but not least it is often needed to check the results of the sample against a GC analysis of a reference sample containing only the suspected substance.
A GC-MS can remove much of this ambiguity, since the mass spectrometer will identify the component's molecular weight. But this still takes time and skill to do properly.
Similarly, most GC analyses are not push-button operations. You cannot simply drop a sample vial into an auto-sampler's tray, push a button and have a computer tell you everything you need to know about the sample. According to the substances one expects to find the operating program must be carefully chosen.
A push-button operation can exist for running similar samples repeatedly, such as in a chemical production environment or for comparing 20 samples from the same experiment to calculate the mean content of the same substance. However, for the kind of investigative work portrayed in books, movies and TV shows this is clearly not the case.
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
used in analytical chemistry
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
for separating
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
and analysing compounds that can be vaporized without decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
In gas chromatography, the mobile phase (or "moving phase") is a carrier gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
, usually an inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
gas such as helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
or an unreactive gas such as nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. The stationary phase is a microscopic layer of liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
on an inert solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
support, inside a piece of glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
or metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
tubing called a column (an homage to the fractionating column
Fractionating column
A fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
used in distillation). The instrument used to perform gas chromatography is called a gas chromatograph (or "aerograph", "gas separator").
The gaseous compounds being analyzed interact with the walls of the column, which is coated with different stationary phases. This causes each compound to elute
Elution
Elution is a term used in analytical and organic chemistry to describe the process of extracting one material from another by washing with a solvent ....
at a different time, known as the retention time of the compound. The comparison of retention times is what gives GC its analytical usefulness.
Gas chromatography is in principle similar to column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
(as well as other forms of chromatography, such as HPLC
High-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
, TLC
Thin layer chromatography
Thin layer chromatography is a chromatography technique used to separate mixtures. Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide, or cellulose...
), but has several notable differences. Firstly, the process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas mobile phase, whereas in column chromatography the stationary phase is a solid and the mobile phase is a liquid. (Hence the full name of the procedure is "Gas–liquid chromatography", referring to the mobile and stationary phases, respectively.) Secondly, the column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled, whereas column chromatography (typically) has no such temperature control. Thirdly, the concentration of a compound in the gas phase is solely a function
Function (mathematics)
In mathematics, a function associates one quantity, the argument of the function, also known as the input, with another quantity, the value of the function, also known as the output. A function assigns exactly one output to each input. The argument and the value may be real numbers, but they can...
of the vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
of the gas.
Gas chromatography is also similar to fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
, since both processes separate the components of a mixture primarily based on boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
(or vapor pressure) differences. However, fractional distillation is typically used to separate components of a mixture on a large scale, whereas GC can be used on a much smaller scale (i.e. microscale).
Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently found in scientific literature. Strictly speaking, GLPC is the most correct terminology, and is thus preferred by many authors.
History
ChromatographyChromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
dates to 1903 in the work of the Russian scientist, Mikhail Semenovich Tswett. German
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
graduate student Fritz Prior developed solid state gas chromatography in 1947. Archer John Porter Martin
Archer John Porter Martin
Archer John Porter Martin, FRS was a British chemist who shared the 1952 Nobel Prize in Chemistry for the invention of partition chromatography with Richard Synge....
, who was awarded the Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
for his work in developing liquid–liquid (1941) and paper (1944) chromatography, laid the foundation for the development of gas chromatography and he later produced liquid-gas chromatography (1950). Erika Cremer
Erika Cremer
Erika Cremer was a German physical chemist and Professor Emeritus at the University of Innsbruck who is regarded as the most important pioneer in gas chromatography, as she first conceived the technique in 1944.-Family:Cremer was from a family of scientists and university professors...
laid the groundwork, and oversaw much of Prior's work.
GC analysis
A gas chromatograph is a chemical analysis instrument for separating chemicals in a complex sample. A gas chromatograph uses a flow-through narrow tube known as the column, through which different chemical constituents of a sample pass in a gas stream (carrier gas, mobile phase) at different rates depending on their various chemical and physical properties and their interaction with a specific column filling, called the stationary phase. As the chemicals exit the end of the column, they are detected and identified electronically. The function of the stationary phase in the column is to separate different components, causing each one to exit the column at a different time (retention time). Other parameters that can be used to alter the order or time of retention are the carrier gas flow rate, column length and the temperature.In a GC analysis, a known volume of gaseous or liquid analyte
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
is injected into the "entrance" (head) of the column, usually using a microsyringe
Syringe
A syringe is a simple pump consisting of a plunger that fits tightly in a tube. The plunger can be pulled and pushed along inside a cylindrical tube , allowing the syringe to take in and expel a liquid or gas through an orifice at the open end of the tube...
(or, solid phase microextraction fibers, or a gas source switching system). As the carrier gas sweeps the analyte molecules through the column, this motion is inhibited by the adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
of the analyte molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s either onto the column walls or onto packing materials in the column. The rate at which the molecules progress along the column depends on the strength of adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
, which in turn depends on the type of molecule and on the stationary phase materials. Since each type of molecule has a different rate of progression, the various components of the analyte mixture are separated as they progress along the column and reach the end of the column at different times (retention time). A detector is used to monitor the outlet stream from the column; thus, the time at which each component reaches the outlet and the amount of that component can be determined. Generally, substances are identified (qualitatively) by the order in which they emerge (elute) from the column and by the retention time of the analyte in the column.
Physical components

Autosamplers
The autosampler providesthe means to introduce a sample automatically into the inlets. Manual insertion of the sample is possible but is no longer common. Automatic insertion provides better reproducibility and time-optimization.
Different kinds of autosamplers exist. Autosamplers can be classified in relation to sample capacity (auto-injectors vs. autosamplers, where auto-injectors can work a small number of samples), to robotic technologies (XYZ robot vs. rotating robot – the most common), or to analysis:
- Liquid
- Static head-space by syringe technology
- Dynamic head-space by transfer-line technology
- Solid phase microextractionSolid phase microextractionSolid-phase microextraction, or SPME, is a sample preparation technique used both in the laboratory and on-site. Developed in the early 1990s at the University of Waterloo by Dr. Pawliszyn's group, it is a simple and inexpensive technique where the use of solvents is not necessary.SPME can be...
(SPME)
Traditionally autosampler manufacturers are different from GC manufacturers and currently no GC manufacturer offers a complete range of autosamplers. Historically, the countries most active in autosampler technology development are the United States, Italy, Switzerland, and the United Kingdom.
Inlets
The column inlet (or injector) provides the means to introduce a sample into a continuous flow of carrier gas. The inlet is a piece of hardware attached to the column head.Common inlet types are:
- S/SL (Split/Splitless) injector; a sample is introduced into a heated small chamber via a syringe through a septum – the heat facilitates volatilization of the sample and sample matrix. The carrier gas then either sweeps the entirety (splitless mode) or a portion (split mode) of the sample into the column. In split mode, a part of the sample/carrier gas mixture in the injection chamber is exhausted through the split vent. Split injection is preferred when working with samples with high analyte concentrations (>0.1%) whereas splitless injection is best suited for trace analysis with low amounts of analytes (<0.01%). In splitless mode the split valve opens after a pre-set amount of time to purge heavier elements that would otherwise contaminate the system. This pre-set (splitless) time should be optimized, the shorter time (e.g., 0.2 min) ensures less tailing but loss in response, the longer time (2 min) increases tailing but also signal.
- On-column inlet; the sample is here introduced directly into the column in its entirety without heat.
- PTV injector; Temperature-programmed sample introduction was first described by Vogt in 1979. Originally Vogt developed the technique as a method for the introduction of large sample volumes (up to 250 µL) in capillary GC. Vogt introduced the sample into the liner at a controlled injection rate. The temperature of the liner was chosen slightly below the boiling point of the solvent. The low-boiling solvent was continuously evaporated and vented through the split line. Based on this technique, Poy developed the Programmed Temperature Vaporising injector; PTV. By introducing the sample at a low initial liner temperature many of the disadvantages of the classic hot injection techniques could be circumvented.
- Gas source inlet or gas switching valve; gaseous samples in collection bottles are connected to what is most commonly a six-port switching valve. The carrier gas flow is not interrupted while a sample can be expanded into a previously evacuated sample loop. Upon switching, the contents of the sample loop are inserted into the carrier gas stream.
- P/T (Purge-and-Trap) system; An inert gas is bubbled through an aqueous sample causing insoluble volatile chemicals to be purged from the matrix. The volatiles are 'trapped' on an absorbent column (known as a trap or concentrator) at ambient temperature. The trap is then heated and the volatiles are directed into the carrier gas stream. Samples requiring preconcentration or purification can be introduced via such a system, usually hooked up to the S/SL port.
The choice of carrier gas (mobile phase) is important, with hydrogen being the most efficient and providing the best separation. However, helium has a larger range of flowrates that are comparable to hydrogen in efficiency, with the added advantage that helium is non-flammable, and works with a greater number of detectors. Therefore, helium is the most common carrier gas used.
Detectoron are the flame ionization detector
Flame ionization detector
A flame ionization detector is a type of gas detector used in gas chromatography. The first flame ionization detector was developed in 1957 by scientists working for the CSIRO in Melbourne, Australia....
(FID) and the thermal conductivity detector
Thermal conductivity detector
The thermal conductivity detector , also known as a Katharometer, is a bulk property detector and a chemical specific detector commonly used in gas-liquid chromatography. This detector senses changes in the thermal conductivity of the column effluent and compares it to a reference flow of carrier gas...
(TCD). Both are sensitive to a wide range of components, and both work over a wide range of concentrations. While TCDs are essentially universal and can be used to detect any component other than the carrier gas (as long as their thermal conductivities are different from that of the carrier gas, at detector temperature), FIDs are sensitive primarily to hydrocarbons, and are more sensitive to them than TCD. However, an FID cannot detect water. Both detectors are also quite robust. Since TCD is non-destructive, it can be operated in-series before an FID (destructive), thus providing complementary detection of the same analytes.
Other detectors are sensitive only to specific types of substances, or work well only in narrower ranges of concentrations. They include:
- catalytic combustion detector (CCD), which measures combustible hydrocarbons and hydrogen.
- discharge ionization detectorDischarge ionization detectorA discharge ionization detector is a type of detector used in gas chromatography.-Principle:A DID is an ion detector which uses a high-voltage electric discharge to produce ions. The spark ionizes helium atoms that are mixed with components as they elute from the GC's column, causing the...
(DID), which uses a high-voltage electric discharge to produce ions. - dry electrolytic conductivity detector (DELCD), which uses an air phase and high temperature (v. Coulsen) to measure chlorinated compounds.
- electron capture detectorElectron capture detectorAn electron capture detector is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by Dr. James E...
(ECD), which uses a radioactive Beta particleBeta particleBeta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
(electron) source to measure the degree of electron capture. - flame photometric detector (FPD)
- flame ionization detectorFlame ionization detectorA flame ionization detector is a type of gas detector used in gas chromatography. The first flame ionization detector was developed in 1957 by scientists working for the CSIRO in Melbourne, Australia....
(FID) - Hall electrolytic conductivity detector (ElCD)
- helium ionization detectorHelium ionization detectorA helium ionization detector is a type of detector used in gas chromatography.-Principle:An HID is an ion detector which uses a radioactive source to produce ions. The radioactive source ionizes helium atoms by bombarding them with emissions. As components elute from the GC's column they are...
(HID) - Nitrogen Phosphorus DetectorNitrogen Phosphorus DetectorThe nitrogen phosphorus detector is a form of gas chromatography in which thermal energy is used to ionize an analyte. With this method, nitrogen and phosphorus can be selectively detected with a sensitivity that is 104 times greater than that for carbon....
(NPD) - Infrared DetectorInfrared detectorAn infrared detector is a photodetector that reacts to infrared radiation. The two main types of detectors are thermal and photonic.The thermal effects of the incident IR radiation can be followed through many temperature dependent phenomena....
(IRD) - mass selective detector (MSD)
- photo-ionization detector (PID)
- pulsed discharge ionization detectorPulsed discharge ionization detectorPDDs utilize a stable, low powered, pulsed DC discharge in helium as an ionization source. Eluants from the column, flowing counter to the flow of helium from the discharge zone, are ionized by photons from the helium discharge...
(PDD) - thermal energy(conductivity) analyzer/detector (TEA/TCD)
- thermionic ionization detector (TID)
Some gas chromatographs are connected to a mass spectrometer which acts as the detector. The combination is known as GC-MS. Some GC-MS are connected to an NMR spectrometer which acts as a backup detector. This combination is known as GC-MS-NMR. Some GC-MS-NMR are connected to an infrared spectrophotometer
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
which acts as a backup detector. This combination is known as GC-MS-NMR-IR. It must, however, be stressed this is very rare as most analyses needed can be concluded via purely GC-MS.
Methods
The method is the collection of conditions in which the GC operates for a given analysis. Method development is the process of determining what conditions are adequate and/or ideal for the analysis required.Conditions which can be varied to accommodate a required analysis include inlet temperature, detector temperature, column temperature and temperature program, carrier gas and carrier gas flow rates, the column's stationary phase, diameter and length, inlet type and flow rates, sample size and injection technique. Depending on the detector(s) (see below) installed on the GC, there may be a number of detector conditions that can also be varied. Some GCs also include valves which can change the route of sample and carrier flow. The timing of the opening and closing of these valves can be important to method development.

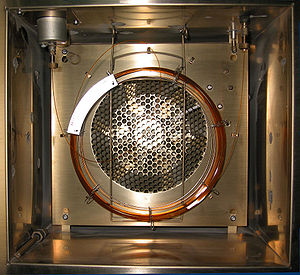
This image above shows the interior of a GeoStrata Technologies Eclipse Gas Chromatograph that runs continuously in three minute cycles. Two valves are used to switch the test gas into the sample loop. After filling the sample loop with test gas, the valves are switched again applying carrier gas pressure to the sample loop and forcing the sample through the Column for separation.
Carrier gas selection and flow rates
Typical carrier gases include helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and air. Which gas to use is usually determined by the detector being used, for example, a DID
Discharge ionization detector
A discharge ionization detector is a type of detector used in gas chromatography.-Principle:A DID is an ion detector which uses a high-voltage electric discharge to produce ions. The spark ionizes helium atoms that are mixed with components as they elute from the GC's column, causing the...
requires helium as the carrier gas. When analyzing gas samples, however, the carrier is sometimes selected based on the sample's matrix, for example, when analyzing a mixture in argon, an argon carrier is preferred, because the argon in the sample does not show up on the chromatogram. Safety and availability can also influence carrier selection, for example, hydrogen is flammable, and high-purity helium can be difficult to obtain in some areas of the world. (See: Helium—occurrence and production.) As a result of helium becoming more scarce, hydrogen is often being substituted for helium as a carrier gas in several applications.
The purity of the carrier gas is also frequently determined by the detector, though the level of sensitivity needed can also play a significant role. Typically, purities of 99.995% or higher are used. The most common purity grades required by modern instruments for the majority of sensitivities are 5.0 grades, or 99.999% pure meaning that there is a total of 10ppm of impurities in the carrier gas that could affect the results. The highest purity grades in common use are 6.0 grades, but the need for detection at very low levels in some forensic and environmental applications has driven the need for carrier gases at 7.0 grade purity and these are now commercially available. Trade names for typical purities include "Zero Grade," "Ultra-High Purity (UHP) Grade," "4.5 Grade" and "5.0 Grade."
The carrier gas linear velocity affects the analysis in the same way that temperature does (see above). The higher the linear velocity the faster the analysis, but the lower the separation between analytes. Selecting the linear velocity is therefore the same compromise between the level of separation and length of analysis as selecting the column temperature. The linear velocity will be implemented by means of the carrier gas flow rate, with regards to the inner diameter of the column.
With GCs made before the 1990s, carrier flow rate was controlled indirectly by controlling the carrier inlet pressure, or "column head pressure." The actual flow rate was measured at the outlet of the column or the detector with an electronic flow meter, or a bubble flow meter, and could be an involved, time consuming, and frustrating process. The pressure setting was not able to be varied during the run, and thus the flow was essentially constant during the analysis. The relation between flow rate and inlet pressure is calculated with Poiseuille's equation for compressible fluids.
Many modern GCs, however, electronically measure the flow rate, and electronically control the carrier gas pressure to set the flow rate. Consequently, carrier pressures and flow rates can be adjusted during the run, creating pressure/flow programs similar to temperature programs.
Stationary compound selection
The polarity
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
of the solute is crucial for the choice of stationary compound, which in an optimal case would have a similar polarity than the solute. Common stationary phases in open tubular columns are cyanopropylphenyl dimethyl polysiloxane, carbowax polyethyleneglycol, biscyanopropyl cyanopropylphenyl polysiloxane and diphenyl dimethyl polysiloxane. For packed columns more options are available.
Inlet types and flow rates
The choice of inlet type and injection technique depends on if the sample is in liquid, gas, adsorbed, or solid form, and on whether a solvent matrix is present that has to be vaporized. Dissolved samples can be introduced directly onto the column via a COC injector, if the conditions are well known; if a solvent matrix has to be vaporized and partially removed, a S/SL injector is used (most common injection technique); gaseous samples (e.g., air cylinders) are usually injected using a gas switching valve system; adsorbed samples (e.g., on adsorbent tubes) are introduced using either an external (on-line or off-line) desorption apparatus such as a purge-and-trap system, or are desorbed in the S/SL injector (SPME applications).
Sample injection

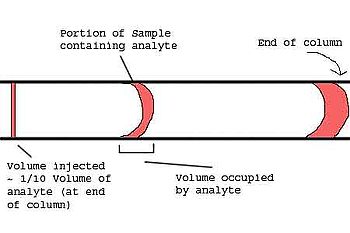
- The amount injected should not overload the column.
- The width of the injected plug should be small compared to the spreading due to the chromatographic process. Failure to comply with this requirement will reduce the separation capability of the column. As a general rule, the volume injected, Vinj, and the volume of the detector cell, Vdet, should be about 1/10 of the volume occupied by the portion of sample containing the molecules of interest (analytes) when they exit the column.
Some general requirements which a good injection technique should fulfill are:
- It should be possible to obtain the column’s optimum separation efficiency.
- It should allow accurate and reproducible injections of small amounts of representative samples.
- It should induce no change in sample composition. It should not exhibit discrimination based on differences in boiling point, polarity, concentration or thermal/catalytic stability.
- It should be applicable for trace analysis as well as for undiluted samples.
Select dimensions of column for corresponding samples and GC System[Limit Temp][Dec-2009]'
Column selection
The choice of column depends on the sample and the active measured. The main chemical attribute regarded when choosing a column is the polarity
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
of the mixture, but functional groups can play a large part in column selection. The polarity of the sample must closely match the polarity of the column stationary phase to increase resolution and separation while reducing run time. The separation and run time also depends on the film thickness (of the stationary phase), the column diameter and the column length.
Column temperature and temperature program

The rate at which a sample passes through the column is directly proportional to the temperature of the column. The higher the column temperature, the faster the sample moves through the column. However, the faster a sample moves through the column, the less it interacts with the stationary phase, and the less the analytes are separated.
In general, the column temperature is selected to compromise between the length of the analysis and the level of separation.
A method which holds the column at the same temperature for the entire analysis is called "isothermal." Most methods, however, increase the column temperature during the analysis, the initial temperature, rate of temperature increase (the temperature "ramp") and final temperature is called the "temperature program."
A temperature program allows analytes that elute early in the analysis to separate adequately, while shortening the time it takes for late-eluting analytes to pass through the column.
Data reduction and analysis
Qualitative analysis:Generally chromatographic data is presented as a graph of detector response (y-axis) against retention time (x-axis), which is called a chromatogram. This provides a spectrum of peaks for a sample representing the analyte
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
s present in a sample eluting from the column at different times. Retention time can be used to identify analytes if the method conditions are constant. Also, the pattern of peaks will be constant for a sample under constant conditions and can identify complex mixtures of analytes. In most modern applications however the GC is connected to a mass spectrometer
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
or similar detector that is capable of identifying the analytes represented by the peaks.
Quantitative analysis:
The area under a peak is proportional to the amount of analyte present in the chromatogram. By calculating the area of the peak using the mathematical function of integration, the concentration of an analyte in the original sample can be determined. Concentration can be calculated using a calibration curve
Calibration curve
In analytical chemistry, a calibration curve is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration...
created by finding the response for a series of concentrations of analyte, or by determining the relative response factor of an analyte. The relative response factor is the expected ratio of an analyte to an internal standard
Internal standard
An internal standard in analytical chemistry is a chemical substance that is added in a constant amount to samples, the blank and calibration standards in a chemical analysis. This substance can then be used for calibration by plotting the ratio of the analyte signal to the internal standard signal...
(or external standard) and is calculated by finding the response of a known amount of analyte and a constant amount of internal standard (a chemical added to the sample at a constant concentration, with a distinct retention time to the analyte).
In most modern GC-MS systems, computer software
Chromatography software
A chromatography software, also known as a Chromatography data system , collects and analyzes chromatographic results delivered by chromatography detectors....
is used to draw and integrate peaks, and match MS
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
spectra to library spectra.
Application
In general, substances that vaporize below ca. 300 °C (and therefore are stable up to that temperature) can be measured quantitatively. The samples are also required to be saltSalt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
-free; they should not contain ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. Very minute amounts of a substance can be measured, but it is often required that the sample must be measured in comparison to a sample containing the pure, suspected substance known as a reference standard
Reference standard
A drug reference standard is a standardized substance which is used as a measurement base for similar substances. Where the exact active substances of a new drug are not known, a reference standard provides a calibrated level of biological effects against which new preparations of the drug can be...
.
Various temperature programs can be used to make the readings more meaningful; for example to differentiate between substances that behave similarly during the GC process.
Professionals working with GC analyze the content of a chemical product, for example in assuring the quality of products in the chemical industry; or measuring toxic substances in soil, air or water. GC is very accurate if used properly and can measure picomoles of a substance in a 1 ml liquid sample, or parts-per-billion concentrations in gaseous samples.
In practical courses at colleges, students sometimes get acquainted to the GC by studying the contents of Lavender
Lavender
The lavenders are a genus of 39 species of flowering plants in the mint family, Lamiaceae. An Old World genus, distributed from Macaronesia across Africa, the Mediterranean, South-West Asia, Arabia, Western Iran and South-East India...
oil or measuring the ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
that is secreted by Nicotiana benthamiana
Nicotiana benthamiana
Nicotiana benthamiana is a close relative of tobacco and species of Nicotiana indigenous to Australia.The herbaceous plant is found amongst rocks on hills and cliffs throughout the northern regions of Australia. Variable in height and habit, the species may be erect and up to 1.5 metres or...
plants after artificially injuring their leaves. These GC analyses hydrocarbons (C2-C40+). In a typical experiment, a packed column is used to separate the light gases, which are then detected with a TCD
Thermal conductivity detector
The thermal conductivity detector , also known as a Katharometer, is a bulk property detector and a chemical specific detector commonly used in gas-liquid chromatography. This detector senses changes in the thermal conductivity of the column effluent and compares it to a reference flow of carrier gas...
. The hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s are separated using a capillary column and detected with an FID
Flame ionization detector
A flame ionization detector is a type of gas detector used in gas chromatography. The first flame ionization detector was developed in 1957 by scientists working for the CSIRO in Melbourne, Australia....
. A complication with light gas analyses that include H2 is that He, which is the most common and most sensitive inert carrier (sensitivity is proportional to molecular mass) has an almost identical thermal conductivity to hydrogen (it is the difference in thermal conductivity between two separate filaments in a Wheatstone Bridge type arrangement that shows when a component has been eluted). For this reason, dual TCD instruments are used with a separate channel for hydrogen that uses nitrogen as a carrier are common. Argon is often used when analysing gas phase chemistry reactions such as F-T synthesis so that a single carrier gas can be used rather than 2 separate ones. The sensitivity is less but this is a tradeoff for simplicity in the gas supply.
GCs in popular culture
Movies, books and TV shows tend to misrepresent the capabilities of gas chromatography and the work done with these instruments.In the U.S. TV show CSI
CSI: Crime Scene Investigation
CSI: Crime Scene Investigation is an American crime drama television series, which premiered on CBS on October 6, 2000. The show was created by Anthony E. Zuiker and produced by Jerry Bruckheimer...
, for example, GCs are used to rapidly identify unknown samples. "This is gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
bought at a Chevron
Chevron Corporation
Chevron Corporation is an American multinational energy corporation headquartered in San Ramon, California, United States and active in more than 180 countries. It is engaged in every aspect of the oil, gas, and geothermal energy industries, including exploration and production; refining,...
station in the past two weeks," the analyst will say fifteen minutes after receiving the sample.
In fact, a typical GC analysis takes much more time; sometimes a single sample must be run more than an hour according to the chosen program; and even more time is needed to "heat out" the column so it is free from the first sample and can be used for the next. Equally, several runs are needed to confirm the results of a study – a GC analysis of a single sample may simply yield a result per chance (see statistical significance
Statistical significance
In statistics, a result is called statistically significant if it is unlikely to have occurred by chance. The phrase test of significance was coined by Ronald Fisher....
).
Also, GC does not positively identify most samples; and not all substances in a sample will necessarily be detected. All a GC truly tells you is at which relative time a component eluted from the column and that the detector was sensitive to it. To make results meaningful, analysts need to know which components at which concentrations are to be expected; and even then a small amount of a substance can hide itself behind a substance having both a higher concentration and the same relative elution time. Last but not least it is often needed to check the results of the sample against a GC analysis of a reference sample containing only the suspected substance.
A GC-MS can remove much of this ambiguity, since the mass spectrometer will identify the component's molecular weight. But this still takes time and skill to do properly.
Similarly, most GC analyses are not push-button operations. You cannot simply drop a sample vial into an auto-sampler's tray, push a button and have a computer tell you everything you need to know about the sample. According to the substances one expects to find the operating program must be carefully chosen.
A push-button operation can exist for running similar samples repeatedly, such as in a chemical production environment or for comparing 20 samples from the same experiment to calculate the mean content of the same substance. However, for the kind of investigative work portrayed in books, movies and TV shows this is clearly not the case.
See also
- Flash gas chromatography
- Thin layer chromatographyThin layer chromatographyThin layer chromatography is a chromatography technique used to separate mixtures. Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide, or cellulose...
- Analytical chemistryAnalytical chemistryAnalytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
- ChromatographyChromatographyChromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
- Gas chromatography-mass spectrometryGas chromatography-mass spectrometryGas chromatography–mass spectrometry is a method that combines the features of gas-liquid chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fire investigation, environmental analysis, explosives investigation,...
- Katharometer
- Standard additionStandard AdditionThe method of standard addition is used in instrumental analysis to determine concentration of a substance in an unknown sample by comparison to a set of samples of known concentration, similar to using a calibration curve...
- Unresolved Complex MixtureUnresolved Complex MixtureUnresolved Complex Mixture is a feature frequently observed in gas chromatographic data of crude oils and extracts from organisms exposed to oil. One reason why it is important to study the nature of UCMs is that some have been shown to contain toxic components,Donkin, P., Smith, E. L. & Rowland,...
- Inverse gas chromatographyInverse gas chromatographyInverse gas chromatography is a physical characterization technique that is used in the analysis of the surfaces of solids. Traditional GC is an analytical technique....

