Fire-safe polymers
Encyclopedia
Fire-safe polymers are polymers that are resistant to degradation
at high temperatures. There is need for fire-resistant polymers in the construction of small, enclosed spaces such as skyscrapers, boats, and airplane cabins. In these tight spaces, ability to escape in the event of a fire
is compromised, increasing fire
risk. In fact, some studies report that about 20% of victims of airplane crashes are killed not by the crash itself but by ensuing fires. Fire-safe polymers also find application as adhesives in aerospace materials, insulation for electronics
, and in military materials such as canvas tenting.
Some fire-safe polymers naturally exhibit an intrinsic resistance to decomposition
, while others are synthesized by incorporating fire-resistant additives and fillers. Current research in developing fire-safe polymers is focused on modifying various properties of the polymers such as ease of ignition
, rate of heat release, and the evolution of smoke and toxic gases. Standard methods for testing polymer
flammability
vary among countries; in the United States common fire tests include the UL 94 small-flame test, the ASTM E 84 Steiner Tunnel, and the ASTM E 622 National Institute of Standards and Technology
(NIST) smoke chamber. Research on developing fire-safe polymers with more desirable properties is concentrated at the University of Massachusetts Amherst
and at the Federal Aviation Administration
where a long-term research program on developing fire-safe polymers was begun in 1995. The Center for UMass/Industry Research on Polymers (CUMIRP) was established in 1980 in Amherst, MA as a concentrated cluster of scientists from both academia and industry for the purpose of polymer
science and engineering research.
of different materials has been a subject of interest since 450 B.C. when Egyptians
attempted to reduce the flammability
of wood by soaking it in potassium aluminum sulfate (alum
). Between 450 B.C. and the early 20th century, other materials used to reduce the flammability of different materials included mixtures of alum
and vinegar
; clay
and hair
; clay
and gypsum
; alum
, ferrous sulfate, and gypsum
; and ammonium chloride
, ammonium phosphate
, borax
, and various acids. These early attempts found application in reducing the flammability of wood for military materials, theater curtains, and other textiles, for example. Important milestones during this early work include the first patent
for a mixture for controlling flammability issued to Obadiah Wyld in 1735, and the first scientific exploration of controlling flammability, which was undertaken by Joseph Louis Gay-Lussac
in 1821.
. The combination of a halogenated paraffin
and antimony oxide
was found to be successful as a fire retardant
for canvas tenting. Syntheses of polymers, such as polyesters, with fire retardant
monomers were also developed around this time. Incorporating flame-resistant additives into polymers became a common and relatively cheap way to reduce the flammability of polymers, while synthesizing intrinsically fire-resistant polymers has remained a more expensive alternative, although the properties of these polymers are usually more efficient at deterring combustion
.
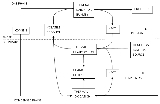
under heat and produce combustible products; thus, they are able to originate and easily propagate fire
(as shown in Figure 1).
The combustion
process begins when heating a polymer
yields volatile
products. If these products are sufficiently concentrated, within the flammability limits, and at a temperature above the ignition temperature, then combustion
proceeds. As long as the heat supplied to the polymer
remains sufficient to sustain its thermal decomposition
at a rate exceeding that required to feed the flame, combustion
will continue.
environment, produce non-combustible products, or add chemicals that would remove fire-propagating radicals
(H and OH), to name a few. These specific chemicals can be added into the polymer
molecules permanently (see Intrinsically Fire-Resistant Polymers) or as additives and fillers (see Flame-Retardant Additives and Fillers).
Role of Oxygen
Oxygen
catalyzes the pyrolysis
of polymers at low concentration and initiates oxidation at high concentration. Transition concentrations are different for different polymers. (e.g., polypropylene
, between 5% and 15%). Additionally, polymers exhibit a structural-dependent relationship with oxygen
. Some structures are intrinsically more sensitive to decomposition
upon reaction with oxygen
. The amount of access that oxygen
has to the surface of the polymer
also plays a role in polymer
combustion
. Oxygen
is better able to interact with the polymer
before a flame has actually been ignited.
of the products.
Role of Pressure
Volatile
products are removed more efficiently under low pressure, which means the stability of the polymer
might have been compromised. Decreased pressure also slows down decomposition
of high boiling products.
are those that are synthesized as intrinsically fire-resistant. However, these types of polymers can be difficult as well as costly to synthesize. Modifying different properties of the polymers can increase their intrinsic fire-resistance; increasing rigidity
or stiffness
, the use of polar
monomers, and/or hydrogen bonding between the polymer
chains can all enhance fire-resistance.
and stability to the polymers. Polyimides, polybenzoxazoles (PBOs), polybenzimidazoles, and polybenzthiazoles (PBTs) are examples of polymers made with aromatic heterocycles (Figure 2).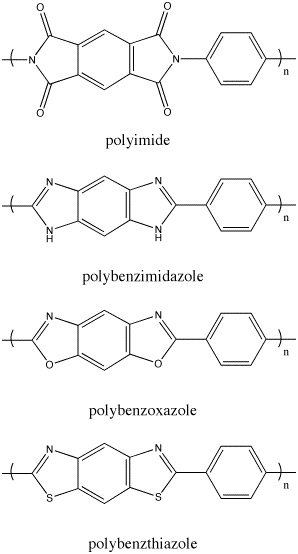 Polymers made with aromatic monomers have a tendency to condense into chars upon combustion
Polymers made with aromatic monomers have a tendency to condense into chars upon combustion
, decreasing the amount of flammable gas that is released. Syntheses of these types of polymers generally employ prepolymers which are further reacted to form the fire-resistant polymers.
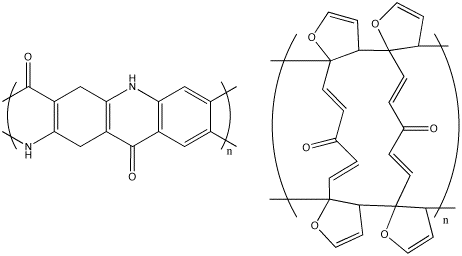 One type of ladder polymer
One type of ladder polymer
links two polymer
chains with periodic covalent bonds. In another type, the ladder polymer
consists of a single chain that is double-stranded. Both types of ladder polymers exhibit good resistance to decomposition
from heat because the chains do not necessarily fall apart if one covalent bond
is broken. However, this makes the processing of ladder polymers difficult because they are not easily melted. These difficulties are compounded because ladder polymers are often highly insoluble.
-nitrogen
, boron
-nitrogen
, and phosphorus
-nitrogen
monomers. The non-burning characteristics of the inorganic components of these polymers contribute to their controlled flammability
. For example, instead of forming toxic, flammable gasses in abundance, polymers prepared with incorporation of cyclotriphosphazene rings give a high char
yield upon combustion
. Polysialates (polymers containing frameworks of aluminum, oxygen
, and silicon
) are another type of inorganic polymer
that can be thermally stable up to temperatures of 1300-1400°C.
. Reactive flame retardants are compounds that are chemically built into the polymer
. They usually contain heteroatoms. Additive flame retardants, on the other hand, are compounds that are not covalently bound to the polymer
; the retardant and the polymer
are just physically mixed together. At present, there are basically six elements
being widely used in this field: boron
, aluminum, phosphorus
, antimony
, chlorine
, and bromine
. One prominent advantage of these types of fire-safe polymers is that they are relatively easy to manufacture.
Natural Fiber
Besides providing satisfactory mechanical properties and renewability, natural fiber
s are easier to obtain and much cheaper than man-made materials. Moreover, they are more environmentally friendly. Recent research focuses on application of different types of fire retardant
s during the manufacturing process as well as applications of fire retardant
s (especially intumescent
coatings) at the finishing stage.
clay in the polymer matrix. Later, organomodified clays, TiO2 nanoparticles, silica nanoparticles, layered double hydroxides
, carbon nanotubes and polyhedral silsesquioxane
s were proved to work as well. Recent research has suggested that combining nanoparticles with traditional fire retardant
s (e.g., intumescent
s) or with surface treatment (e.g., plasma treatment) effectively decreases flammability.
, flame-retardant additives and fillers have disadvantages as well. Their poor compatibility, high volatility
and other deleterious effects can change properties of polymers. Besides, addition of many fire-retardants produces soot
and carbon monoxide
during combustion
. Halogen
-containing materials cause even more concerns on environmental pollution
.
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
at high temperatures. There is need for fire-resistant polymers in the construction of small, enclosed spaces such as skyscrapers, boats, and airplane cabins. In these tight spaces, ability to escape in the event of a fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
is compromised, increasing fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
risk. In fact, some studies report that about 20% of victims of airplane crashes are killed not by the crash itself but by ensuing fires. Fire-safe polymers also find application as adhesives in aerospace materials, insulation for electronics
Electronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
, and in military materials such as canvas tenting.
Some fire-safe polymers naturally exhibit an intrinsic resistance to decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
, while others are synthesized by incorporating fire-resistant additives and fillers. Current research in developing fire-safe polymers is focused on modifying various properties of the polymers such as ease of ignition
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
, rate of heat release, and the evolution of smoke and toxic gases. Standard methods for testing polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
flammability
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
vary among countries; in the United States common fire tests include the UL 94 small-flame test, the ASTM E 84 Steiner Tunnel, and the ASTM E 622 National Institute of Standards and Technology
National Institute of Standards and Technology
The National Institute of Standards and Technology , known between 1901 and 1988 as the National Bureau of Standards , is a measurement standards laboratory, otherwise known as a National Metrological Institute , which is a non-regulatory agency of the United States Department of Commerce...
(NIST) smoke chamber. Research on developing fire-safe polymers with more desirable properties is concentrated at the University of Massachusetts Amherst
University of Massachusetts Amherst
The University of Massachusetts Amherst is a public research and land-grant university in Amherst, Massachusetts, United States and the flagship of the University of Massachusetts system...
and at the Federal Aviation Administration
Federal Aviation Administration
The Federal Aviation Administration is the national aviation authority of the United States. An agency of the United States Department of Transportation, it has authority to regulate and oversee all aspects of civil aviation in the U.S...
where a long-term research program on developing fire-safe polymers was begun in 1995. The Center for UMass/Industry Research on Polymers (CUMIRP) was established in 1980 in Amherst, MA as a concentrated cluster of scientists from both academia and industry for the purpose of polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
science and engineering research.
Early History
Controlling the flammabilityFlammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
of different materials has been a subject of interest since 450 B.C. when Egyptians
Egyptians
Egyptians are nation an ethnic group made up of Mediterranean North Africans, the indigenous people of Egypt.Egyptian identity is closely tied to geography. The population of Egypt is concentrated in the lower Nile Valley, the small strip of cultivable land stretching from the First Cataract to...
attempted to reduce the flammability
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
of wood by soaking it in potassium aluminum sulfate (alum
Alum
Alum is both a specific chemical compound and a class of chemical compounds. The specific compound is the hydrated potassium aluminium sulfate with the formula KAl2.12H2O. The wider class of compounds known as alums have the related empirical formula, AB2.12H2O.-Chemical properties:Alums are...
). Between 450 B.C. and the early 20th century, other materials used to reduce the flammability of different materials included mixtures of alum
Alum
Alum is both a specific chemical compound and a class of chemical compounds. The specific compound is the hydrated potassium aluminium sulfate with the formula KAl2.12H2O. The wider class of compounds known as alums have the related empirical formula, AB2.12H2O.-Chemical properties:Alums are...
and vinegar
Vinegar
Vinegar is a liquid substance consisting mainly of acetic acid and water, the acetic acid being produced through the fermentation of ethanol by acetic acid bacteria. Commercial vinegar is produced either by fast or slow fermentation processes. Slow methods generally are used with traditional...
; clay
Clay
Clay is a general term including many combinations of one or more clay minerals with traces of metal oxides and organic matter. Geologic clay deposits are mostly composed of phyllosilicate minerals containing variable amounts of water trapped in the mineral structure.- Formation :Clay minerals...
and hair
Hair
Hair is a filamentous biomaterial, that grows from follicles found in the dermis. Found exclusively in mammals, hair is one of the defining characteristics of the mammalian class....
; clay
Clay
Clay is a general term including many combinations of one or more clay minerals with traces of metal oxides and organic matter. Geologic clay deposits are mostly composed of phyllosilicate minerals containing variable amounts of water trapped in the mineral structure.- Formation :Clay minerals...
and gypsum
Gypsum
Gypsum is a very soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is found in alabaster, a decorative stone used in Ancient Egypt. It is the second softest mineral on the Mohs Hardness Scale...
; alum
Alum
Alum is both a specific chemical compound and a class of chemical compounds. The specific compound is the hydrated potassium aluminium sulfate with the formula KAl2.12H2O. The wider class of compounds known as alums have the related empirical formula, AB2.12H2O.-Chemical properties:Alums are...
, ferrous sulfate, and gypsum
Gypsum
Gypsum is a very soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is found in alabaster, a decorative stone used in Ancient Egypt. It is the second softest mineral on the Mohs Hardness Scale...
; and ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
, ammonium phosphate
Ammonium phosphate
Ammonium phosphate is the salt of ammonia and phosphoric acid. It has the formula 3PO4 and consists of ammonium cations and phosphate anion. It is obtained as a crystalline powder upon mixing concentrated solutions of ammonia and phosphoric acid, or on the addition of excess of ammonia to the...
, borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
, and various acids. These early attempts found application in reducing the flammability of wood for military materials, theater curtains, and other textiles, for example. Important milestones during this early work include the first patent
Patent
A patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
for a mixture for controlling flammability issued to Obadiah Wyld in 1735, and the first scientific exploration of controlling flammability, which was undertaken by Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
in 1821.
Developments Since WWII
Research on fire-retardant polymers was bolstered by the need for new types of synthetic polymers in World War IIWorld War II
World War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
. The combination of a halogenated paraffin
Paraffin
In chemistry, paraffin is a term that can be used synonymously with "alkane", indicating hydrocarbons with the general formula CnH2n+2. Paraffin wax refers to a mixture of alkanes that falls within the 20 ≤ n ≤ 40 range; they are found in the solid state at room temperature and begin to enter the...
and antimony oxide
Antimony oxide
Antimony oxide may refer to any of the following:*Diantimony tetroxide, Sb2O4*Antimony trioxide, Sb2O3*Antimony pentoxide, Sb2O5...
was found to be successful as a fire retardant
Fire retardant
A fire retardant is a substance other than water that reduces flammability of fuels or delays their combustion. This typically refers to chemical retardants but may also include substances that work by physical action, such as cooling the fuels; examples of these include fire-fighting foams and...
for canvas tenting. Syntheses of polymers, such as polyesters, with fire retardant
Fire retardant
A fire retardant is a substance other than water that reduces flammability of fuels or delays their combustion. This typically refers to chemical retardants but may also include substances that work by physical action, such as cooling the fuels; examples of these include fire-fighting foams and...
monomers were also developed around this time. Incorporating flame-resistant additives into polymers became a common and relatively cheap way to reduce the flammability of polymers, while synthesizing intrinsically fire-resistant polymers has remained a more expensive alternative, although the properties of these polymers are usually more efficient at deterring combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
.
General Mechanistic Scheme
Traditional polymers decomposeChemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
under heat and produce combustible products; thus, they are able to originate and easily propagate fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
(as shown in Figure 1).
The combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
process begins when heating a polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
yields volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
products. If these products are sufficiently concentrated, within the flammability limits, and at a temperature above the ignition temperature, then combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
proceeds. As long as the heat supplied to the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
remains sufficient to sustain its thermal decomposition
Thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes....
at a rate exceeding that required to feed the flame, combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
will continue.
Purpose and Methods of Fire-Retardant Systems
The purpose is to control heat below the critical level. To achieve this, one can create an endothermicEndothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
environment, produce non-combustible products, or add chemicals that would remove fire-propagating radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
(H and OH), to name a few. These specific chemicals can be added into the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
molecules permanently (see Intrinsically Fire-Resistant Polymers) or as additives and fillers (see Flame-Retardant Additives and Fillers).
Role of OxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
OxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
catalyzes the pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
of polymers at low concentration and initiates oxidation at high concentration. Transition concentrations are different for different polymers. (e.g., polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
, between 5% and 15%). Additionally, polymers exhibit a structural-dependent relationship with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. Some structures are intrinsically more sensitive to decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
upon reaction with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. The amount of access that oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
has to the surface of the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
also plays a role in polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. Oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
is better able to interact with the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
before a flame has actually been ignited.
Role of Heating Rate
In most cases, results from a typical heating rate (e.g. 10℃/min for mechanical thermal degradation studies) do not differ significantly from those obtained at higher heating rates. The extent of reaction can, however, be influenced by the heating rate. For example, some reactions may not occur with a low heating rate due to evaporationEvaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
of the products.
Role of PressurePressurePressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
VolatileVolatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
products are removed more efficiently under low pressure, which means the stability of the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
might have been compromised. Decreased pressure also slows down decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
of high boiling products.
Intrinsically Fire-Resistant Polymers
The polymers that are most efficient at resisting combustionCombustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
are those that are synthesized as intrinsically fire-resistant. However, these types of polymers can be difficult as well as costly to synthesize. Modifying different properties of the polymers can increase their intrinsic fire-resistance; increasing rigidity
Rigidity
Rigid or rigidity may refer to:*Stiffness, the property of a solid body to resist deformation, which is sometimes referred to as rigidity*Structural rigidity, a mathematical theory of the stiffness of ensembles of rigid objects connected by hinges...
or stiffness
Stiffness
Stiffness is the resistance of an elastic body to deformation by an applied force along a given degree of freedom when a set of loading points and boundary conditions are prescribed on the elastic body.-Calculations:...
, the use of polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
monomers, and/or hydrogen bonding between the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
chains can all enhance fire-resistance.
Linear, Single-Stranded Polymers With Cyclic Aromatic Components
Most intrinsically fire-resistant polymers are made by incorporation of aromatic cycles or heterocycles, which lend rigidityRigidity
Rigid or rigidity may refer to:*Stiffness, the property of a solid body to resist deformation, which is sometimes referred to as rigidity*Structural rigidity, a mathematical theory of the stiffness of ensembles of rigid objects connected by hinges...
and stability to the polymers. Polyimides, polybenzoxazoles (PBOs), polybenzimidazoles, and polybenzthiazoles (PBTs) are examples of polymers made with aromatic heterocycles (Figure 2).

Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
, decreasing the amount of flammable gas that is released. Syntheses of these types of polymers generally employ prepolymers which are further reacted to form the fire-resistant polymers.
Ladder Polymers
Ladder polymers are a subclass of polymers made with aromatic cycles or heterocycles. Ladder polymers generally have one of two types of general structures, as shown in Figure 3.
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
links two polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
chains with periodic covalent bonds. In another type, the ladder polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
consists of a single chain that is double-stranded. Both types of ladder polymers exhibit good resistance to decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
from heat because the chains do not necessarily fall apart if one covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
is broken. However, this makes the processing of ladder polymers difficult because they are not easily melted. These difficulties are compounded because ladder polymers are often highly insoluble.
Inorganic and Semiorganic Polymers
Inorganic and semiorganic polymers often employ siliconSilicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
-nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
-nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, and phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
-nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
monomers. The non-burning characteristics of the inorganic components of these polymers contribute to their controlled flammability
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
. For example, instead of forming toxic, flammable gasses in abundance, polymers prepared with incorporation of cyclotriphosphazene rings give a high char
Char
Char is the solid material that remains after light gases and tar coal tar have been driven out or released from a carbonaceous material during the initial stage of combustion, which is known as carbonization, charring, devolatilization or pyrolysis.Further stages of efficient combustion are...
yield upon combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. Polysialates (polymers containing frameworks of aluminum, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, and silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
) are another type of inorganic polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
that can be thermally stable up to temperatures of 1300-1400°C.
Flame-Retardant Additives and Fillers
Additives are divided into two basic types depending on the interaction of the additive and polymerPolymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
. Reactive flame retardants are compounds that are chemically built into the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
. They usually contain heteroatoms. Additive flame retardants, on the other hand, are compounds that are not covalently bound to the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
; the retardant and the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
are just physically mixed together. At present, there are basically six elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
being widely used in this field: boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, aluminum, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, and bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
. One prominent advantage of these types of fire-safe polymers is that they are relatively easy to manufacture.
Natural FiberNatural fiberFibers or fibres are a class of hair-like materials that are continuous filaments or are in discrete elongated pieces, similar to pieces of thread. They can be spun into filaments, thread, or rope. They can be used as a component of composite materials. They can also be matted into sheets to...
-Containing Composites
Besides providing satisfactory mechanical properties and renewability, natural fiberNatural fiber
Fibers or fibres are a class of hair-like materials that are continuous filaments or are in discrete elongated pieces, similar to pieces of thread. They can be spun into filaments, thread, or rope. They can be used as a component of composite materials. They can also be matted into sheets to...
s are easier to obtain and much cheaper than man-made materials. Moreover, they are more environmentally friendly. Recent research focuses on application of different types of fire retardant
Fire retardant
A fire retardant is a substance other than water that reduces flammability of fuels or delays their combustion. This typically refers to chemical retardants but may also include substances that work by physical action, such as cooling the fuels; examples of these include fire-fighting foams and...
s during the manufacturing process as well as applications of fire retardant
Fire retardant
A fire retardant is a substance other than water that reduces flammability of fuels or delays their combustion. This typically refers to chemical retardants but may also include substances that work by physical action, such as cooling the fuels; examples of these include fire-fighting foams and...
s (especially intumescent
Intumescent
An intumescent is a substance which swells as a result of heat exposure, thus increasing in volume, and decreasing in density. Intumescents are typically used in passive fire protection and, in America, require listing and approval use and compliance in their installed configurations in order to...
coatings) at the finishing stage.
Nanocomposites
Nanocomposites have become a hotspot in the research of fire-safe polymers because of their relatively low cost and high flexibility for multifunctional properties. Gilman and colleagues did the pioneering work by demonstrating the improvement of fire-retardancy by having nanodispersed montmorilloniteMontmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that typically form in microscopic crystals, forming a clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite family, is a 2:1 clay, meaning that it has 2 tetrahedral sheets sandwiching a central...
clay in the polymer matrix. Later, organomodified clays, TiO2 nanoparticles, silica nanoparticles, layered double hydroxides
Layered double hydroxides
Layered double hydroxides comprise an unusual class of layered materials with positively charged layers and charge balancing anions located in the interlayer region. This is unusual in solid state chemistry: many more families of materials have negatively charged layers and cations in the...
, carbon nanotubes and polyhedral silsesquioxane
Silsesquioxane
200px|thumbnail|right|Figure 1: Silsesquioxane Cage StructureA silsesquioxane is a compound with the empirical chemical formula RSiO3/2 where Si is the element silicon, O is oxygen and R is either hydrogen or an alkyl, alkene, aryl, arylene group.These materials can be used as a support for...
s were proved to work as well. Recent research has suggested that combining nanoparticles with traditional fire retardant
Fire retardant
A fire retardant is a substance other than water that reduces flammability of fuels or delays their combustion. This typically refers to chemical retardants but may also include substances that work by physical action, such as cooling the fuels; examples of these include fire-fighting foams and...
s (e.g., intumescent
Intumescent
An intumescent is a substance which swells as a result of heat exposure, thus increasing in volume, and decreasing in density. Intumescents are typically used in passive fire protection and, in America, require listing and approval use and compliance in their installed configurations in order to...
s) or with surface treatment (e.g., plasma treatment) effectively decreases flammability.
Problems With Additives and Fillers
Although effective at reducing flammabilityFlammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
, flame-retardant additives and fillers have disadvantages as well. Their poor compatibility, high volatility
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
and other deleterious effects can change properties of polymers. Besides, addition of many fire-retardants produces soot
Soot
Soot is a general term that refers to impure carbon particles resulting from the incomplete combustion of a hydrocarbon. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolyzed fuel particles such as cenospheres,...
and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
during combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. Halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
-containing materials cause even more concerns on environmental pollution
Pollution
Pollution is the introduction of contaminants into a natural environment that causes instability, disorder, harm or discomfort to the ecosystem i.e. physical systems or living organisms. Pollution can take the form of chemical substances or energy, such as noise, heat or light...
.
See also
- Plastics
- FireproofingFireproofingFireproofing, a passive fire protection measure, refers to the act of making materials or structures more resistant to fire, or to those materials themselves, or the act of applying such materials. Applying a certification listed fireproofing system to certain structures allows these to have a...
- Phenol formaldehyde resinPhenol formaldehyde resinPhenol formaldehyde resins include synthetic thermosetting resins such as obtained by the reaction of phenols with formaldehyde. Sometimes the precursors include other aldehydes or other phenol. Phenolic resins are mainly used in the production of circuit boards...
- University of Massachusetts AmherstUniversity of Massachusetts AmherstThe University of Massachusetts Amherst is a public research and land-grant university in Amherst, Massachusetts, United States and the flagship of the University of Massachusetts system...
- PyrolysisPyrolysisPyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
- CombustionCombustionCombustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...

