Extrinsic semiconductor
Encyclopedia
An extrinsic semiconductor is a semiconductor
that has been doped, that is, into which a doping agent
has been introduced, giving it different electrical properties than the intrinsic (pure) semiconductor
.
Doping involves adding dopant atoms to an intrinsic semiconductor, which changes the electron
and hole
carrier concentrations of the semiconductor at thermal equilibrium
. Dominant carrier concentrations in an extrinsic semiconductor classify it as either an n-type
or p-type
semiconductor. The electrical properties of extrinsic semiconductors make them essential components of many electronic devices.
is the process that changes an intrinsic semiconductor to an extrinsic semiconductor. During doping, impurity atoms are introduced to an intrinsic semiconductor. Impurity atoms are atoms of a different element than the atoms of the intrinsic semiconductor. Impurity atoms act as either donors
or acceptors
to the intrinsic semiconductor, changing the electron and hole concentrations of the semiconductor. Impurity atoms are classified as donor or acceptor atoms based on the effect they have on the intrinsic semiconductor.
Donor impurity atoms have more valence electron
s than the atoms they replace in the intrinsic semiconductor lattice. Donor impurities "donate" their extra valence electrons to a semiconductor's conduction band, providing excess electrons to the intrinsic semiconductor. Excess electrons increase the electron carrier concentration (n0) of the semiconductor, making it n-type.
Acceptor impurity atoms have less valence electrons than the atoms they replace in the intrinsic semiconductor. They "accept" electrons from the semiconductor's valence band. This provides excess holes to the intrinsic semiconductor. Excess holes increase the hole carrier concentration (p0) of the semiconductor, creating a p-type semiconductor.
Semiconductors and dopant atoms are defined by the column of the periodic table of elements they fall in. The column definition of the semiconductor determines how many valence electrons its atoms have and whether dopant atoms act as the semiconductor's donors or acceptors.
Group IV semiconductors use group V
atoms as donors and group III
atoms as acceptors.
Group III-V semiconductors, the compound semiconductor
s, use group VI
atoms as donors and group II
atoms as acceptors. Group III-V semiconductors can also use group IV
atoms as either donors or acceptors. When a group IV atom replaces the group III element in the semiconductor lattice, the group IV atom acts as a donor. Conversely, when a group IV atom replaces the group V element, the group IV atom acts as an acceptor. Group IV atoms can act as both donors and acceptors; therefore, they are known as amphoteric
impurities.
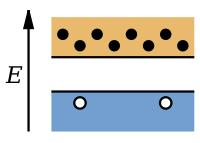 Extrinsic semiconductors with a larger electron concentration than hole concentration are known as n-type semiconductor
Extrinsic semiconductors with a larger electron concentration than hole concentration are known as n-type semiconductor
s. The phrase 'n-type' comes from the negative charge of the electron. In n-type semiconductors, electrons are the majority carriers and holes are the minority carriers. N-type semiconductors are created by doping an intrinsic semiconductor with donor impurities. In an n-type semiconductor, the Fermi energy level
is greater than that of the intrinsic semiconductor and lies closer to the conduction band
than the valence band
.
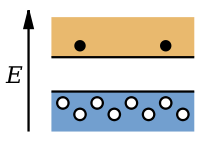 As opposed to n-type semiconductors, p-type semiconductor
As opposed to n-type semiconductors, p-type semiconductor
s have a larger hole concentration than electron concentration. The phrase 'p-type' refers to the positive charge of the hole. In p-type semiconductors, holes are the majority carriers and electrons are the minority carriers. P-type semiconductors are created by doping an intrinsic semiconductor with acceptor impurities. P-type semiconductors have Fermi energy levels below the intrinsic Fermi energy level. The Fermi energy level lies closer to the valence band than the conduction band in a p-type semiconductor.
(devices that allow current in only one direction) consists of p-type and n-type semiconductors placed in junction
with one another. Currently, most semiconductor diodes use doped silicon or germanium.
Transistors (devices that enable current switching) also make use of extrinsic semiconductors. Bipolar junction transistors (BJT) are one type of transistor. The most common BJTs are NPN and PNP type. NPN transistors have two layers of n-type semiconductors sandwiching a p-type semiconductor. PNP transistors have two layers of p-type semiconductors sandwiching an n-type semiconductor.
Field-effect transistor
s (FET) are another type of transistor implementing extrinsic semiconductors. As opposed to BJTs, they are unipolar and considered either N-channel or P-channel. FETs are broken into two families, junction gate FET
(JFET) and insulated gate FET (IGFET).
Other devices implementing the extrinsic semiconductor:
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
that has been doped, that is, into which a doping agent
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
has been introduced, giving it different electrical properties than the intrinsic (pure) semiconductor
Intrinsic semiconductor
An intrinsic semiconductor, also called an undoped semiconductor or i-type semiconductor, is a pure semiconductor without any significant dopant species present. The number of charge carriers is therefore determined by the properties of the material itself instead of the amount of impurities...
.
Doping involves adding dopant atoms to an intrinsic semiconductor, which changes the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
and hole
Electron hole
An electron hole is the conceptual and mathematical opposite of an electron, useful in the study of physics, chemistry, and electrical engineering. The concept describes the lack of an electron at a position where one could exist in an atom or atomic lattice...
carrier concentrations of the semiconductor at thermal equilibrium
Thermal equilibrium
Thermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
. Dominant carrier concentrations in an extrinsic semiconductor classify it as either an n-type
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
or p-type
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
semiconductor. The electrical properties of extrinsic semiconductors make them essential components of many electronic devices.
Semiconductor doping
Semiconductor dopingDoping (semiconductor)
In semiconductor production, doping intentionally introduces impurities into an extremely pure semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic...
is the process that changes an intrinsic semiconductor to an extrinsic semiconductor. During doping, impurity atoms are introduced to an intrinsic semiconductor. Impurity atoms are atoms of a different element than the atoms of the intrinsic semiconductor. Impurity atoms act as either donors
Donor (semiconductors)
In semiconductor physics, a donor is a dopant atom that, when added to a semiconductor, can form n-type regions.For example, when silicon , having four valence electrons, needs to be doped as an n-type semiconductor, elements from group V like phosphorus or arsenic can be used because they have...
or acceptors
Acceptor (semiconductors)
In semiconductor physics, an acceptor is a dopant atom that when added to a semiconductor can form p-type regions.For example, when silicon , having four valence electrons, needs to be doped as a p-type semiconductor, elements from group III like boron or aluminium , having three valence...
to the intrinsic semiconductor, changing the electron and hole concentrations of the semiconductor. Impurity atoms are classified as donor or acceptor atoms based on the effect they have on the intrinsic semiconductor.
Donor impurity atoms have more valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s than the atoms they replace in the intrinsic semiconductor lattice. Donor impurities "donate" their extra valence electrons to a semiconductor's conduction band, providing excess electrons to the intrinsic semiconductor. Excess electrons increase the electron carrier concentration (n0) of the semiconductor, making it n-type.
Acceptor impurity atoms have less valence electrons than the atoms they replace in the intrinsic semiconductor. They "accept" electrons from the semiconductor's valence band. This provides excess holes to the intrinsic semiconductor. Excess holes increase the hole carrier concentration (p0) of the semiconductor, creating a p-type semiconductor.
Semiconductors and dopant atoms are defined by the column of the periodic table of elements they fall in. The column definition of the semiconductor determines how many valence electrons its atoms have and whether dopant atoms act as the semiconductor's donors or acceptors.
Group IV semiconductors use group V
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
atoms as donors and group III
Boron group
The boron group is the series of elements in group 13 of the periodic table, comprising boron , aluminium , gallium , indium , thallium , and ununtrium . The elements in the boron group are characterized by having three electrons in their outer energy levels...
atoms as acceptors.
Group III-V semiconductors, the compound semiconductor
Compound semiconductor
A compound semiconductor is a semiconductor compound composed of elements from two or more different groups of the periodic table . These semiconductors typically form in groups 13-16 ,...
s, use group VI
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
atoms as donors and group II
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
atoms as acceptors. Group III-V semiconductors can also use group IV
Carbon group
The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
atoms as either donors or acceptors. When a group IV atom replaces the group III element in the semiconductor lattice, the group IV atom acts as a donor. Conversely, when a group IV atom replaces the group V element, the group IV atom acts as an acceptor. Group IV atoms can act as both donors and acceptors; therefore, they are known as amphoteric
Amphoterism
In chemistry, an amphoteric species is a molecule or ion that can react as an acid as well as a base. The word is derived from the Greek word amphoteroi meaning "both"...
impurities.
| Intrinsic semiconductor | Donor atoms | Acceptor atoms | |
|---|---|---|---|
| Group IV semiconductors | Silicon Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... , Germanium Germanium Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon.... |
Phosphorus Phosphorus Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks... , Arsenic Arsenic Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid... |
Boron Boron Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the... , Aluminium Aluminium Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances.... |
| Group III-V semiconductors | Aluminum phosphide, Aluminum arsenide, Gallium arsenide, Gallium nitride | Selenium Selenium Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium... , Tellurium, Silicon Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... , Germanium Germanium Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon.... |
Beryllium Beryllium Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl... , Zinc Zinc Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2... , Cadmium Cadmium Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low... , Silicon Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... , Germanium Germanium Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon.... |
N-type semiconductors
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
s. The phrase 'n-type' comes from the negative charge of the electron. In n-type semiconductors, electrons are the majority carriers and holes are the minority carriers. N-type semiconductors are created by doping an intrinsic semiconductor with donor impurities. In an n-type semiconductor, the Fermi energy level
Fermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy of the highest occupied quantum state in a system of fermions at absolute zero temperature....
is greater than that of the intrinsic semiconductor and lies closer to the conduction band
Conduction band
In the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
than the valence band
Valence band
In solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
.
P-type semiconductors
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
s have a larger hole concentration than electron concentration. The phrase 'p-type' refers to the positive charge of the hole. In p-type semiconductors, holes are the majority carriers and electrons are the minority carriers. P-type semiconductors are created by doping an intrinsic semiconductor with acceptor impurities. P-type semiconductors have Fermi energy levels below the intrinsic Fermi energy level. The Fermi energy level lies closer to the valence band than the conduction band in a p-type semiconductor.
Utilization of extrinsic semiconductors
Extrinsic semiconductors are components of many common electrical devices. A semiconductor diodeDiode
In electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
(devices that allow current in only one direction) consists of p-type and n-type semiconductors placed in junction
P-n junction
A p–n junction is formed at the boundary between a P-type and N-type semiconductor created in a single crystal of semiconductor by doping, for example by ion implantation, diffusion of dopants, or by epitaxy .If two separate pieces of material were used, this would...
with one another. Currently, most semiconductor diodes use doped silicon or germanium.
Transistors (devices that enable current switching) also make use of extrinsic semiconductors. Bipolar junction transistors (BJT) are one type of transistor. The most common BJTs are NPN and PNP type. NPN transistors have two layers of n-type semiconductors sandwiching a p-type semiconductor. PNP transistors have two layers of p-type semiconductors sandwiching an n-type semiconductor.
Field-effect transistor
Field-effect transistor
The field-effect transistor is a transistor that relies on an electric field to control the shape and hence the conductivity of a channel of one type of charge carrier in a semiconductor material. FETs are sometimes called unipolar transistors to contrast their single-carrier-type operation with...
s (FET) are another type of transistor implementing extrinsic semiconductors. As opposed to BJTs, they are unipolar and considered either N-channel or P-channel. FETs are broken into two families, junction gate FET
JFET
The junction gate field-effect transistor is the simplest type of field-effect transistor. It can be used as an electronically-controlled switch or as a voltage-controlled resistance. Electric charge flows through a semiconducting channel between "source" and "drain" terminals...
(JFET) and insulated gate FET (IGFET).
Other devices implementing the extrinsic semiconductor:
- LaserLaserA laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
s - Solar cellSolar cellA solar cell is a solid state electrical device that converts the energy of light directly into electricity by the photovoltaic effect....
s - PhotodetectorPhotodetectorPhotosensors or photodetectors are sensors of light or other electromagnetic energy. There are several varieties:*Active pixel sensors are image sensors consisting of an integrated circuit that contains an array of pixel sensors, each pixel containing a both a light sensor and an active amplifier...
s - Light-emitting diodeLight-emitting diodeA light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
s - Thyristors
See also
- Intrinsic semiconductorIntrinsic semiconductorAn intrinsic semiconductor, also called an undoped semiconductor or i-type semiconductor, is a pure semiconductor without any significant dopant species present. The number of charge carriers is therefore determined by the properties of the material itself instead of the amount of impurities...
- Doping (semiconductor)Doping (semiconductor)In semiconductor production, doping intentionally introduces impurities into an extremely pure semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic...
- List of semiconductor materials

