
Doping (semiconductor)
Encyclopedia
In semiconductor
production, doping intentionally introduces impurities into an extremely pure (also referred to as intrinsic) semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic
. A semiconductor doped to such high levels that it acts more like a conductor than a semiconductor is referred to as degenerate
.
In the context of phosphor
s and scintillator
s, doping is better known as activation
.
working at Sperry Gyroscope Company during World War II
. The demands of his work on radar
denied Woodyard the opportunity to pursue research on semiconductor doping. However, after the war ended, his patent
proved the grounds of extensive litigation by Sperry Rand. Related work was performed at Bell Labs
by Gordon K. Teal
and Morgan Sparks
.
s are added as the (usually silicon
) boule
is grown, giving each wafer
an almost uniform initial doping. To define circuit elements, selected areas — typically controlled by photolithography
— are further doped by such processes as diffusion
and ion implantation
, the latter method being more popular in large production runs because of increased controllability.
Small numbers of dopant atoms can change the ability of a semiconductor to conduct electricity. When on the order of one dopant atom is added per 100 million atoms, the doping is said to be low or light. When many more dopant atoms are added, on the order of one per ten thousand atoms, the doping is referred to as heavy or high. This is often shown as n+ for n-type
doping or p+ for p-type
doping. (See the article on semiconductors for a more detailed description of the doping mechanism.)
is called "Group IV", not "Group 14".)
For the Group IV
semiconductors such as silicon
, germanium
, and silicon carbide
, the most common dopants are acceptors
from Group III
or donors
from Group V
elements. Boron
, arsenic
, phosphorus
, and occasionally gallium
are used to dope silicon. Boron is the p-type dopant
of choice for silicon integrated circuit production because it diffuses at a rate that makes junction depths easily controllable. Phosphorus is typically used for bulk-doping of silicon wafers, while arsenic is used to diffuse junctions, because it diffuses more slowly than phosphorus and is thus more controllable.
By doping pure silicon with Group V
elements such as phosphorus, extra valence electrons are added that become unbonded from individual atoms and allow the compound to be an electrically conductive n-type semiconductor
. Doping with Group III
elements, which are missing the fourth valence electron, creates "broken bonds" (holes) in the silicon lattice that are free to move. The result is an electrically conductive p-type semiconductor
. In this context, a Group V
element is said to behave as an electron donor, and a group III
element as an acceptor. This is a key concept in the physics of a diode
.
Very heavily doped semiconductor behaves more like a good conductor (metal) and thus exhibits more linear positive thermal coefficient. Such effect is used for instance in sensistor
s. Lower dosage of doping is used in other types (NTC or PTC) thermistor
s.
in the vast majority of semiconductor devices. Partial compensation, where donors outnumber acceptors or vice versa, allows device makers to repeatedly reverse the type of a given portion of the material by applying successively higher doses of dopants.
Although compensation can be used to increase or decrease the number of donors or acceptors, the electron and hole mobility
is always decreased by compensation because mobility is affected by the sum of the donor and acceptor ions.
s can be doped by adding chemical reactants to oxidize, or sometimes reduce, the system so that electrons are pushed into the conducting orbitals
within the already potentially conducting system. There are two primary methods of doping a conductive polymer, both of which use an oxidation-reduction (i.e., redox
) process.
N-doping is much less common because the Earth's atmosphere
is oxygen
-rich, thus creating an oxidizing environment. An electron-rich, n-doped polymer will react immediately with elemental oxygen to de-dope (i.e., reoxidize to the neutral state) the polymer. Thus, chemical n-doping must be performed in an environment of inert gas
(e.g., argon
). Electrochemical n-doping is far more common in research, because it is easier to exclude oxygen from a solvent
in a sealed flask
. However, it is unlikely that n-doped conductive polymers are available commercially.
transmutation
doping (NTD) is an unusual doping method for special applications. Most commonly, it is used to dope silicon n-type in high-power electronics. It is based on the conversion of the Si-30 isotope into phosphorus
atom by neutron absorption as follows:
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
production, doping intentionally introduces impurities into an extremely pure (also referred to as intrinsic) semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic
Extrinsic semiconductor
An extrinsic semiconductor is a semiconductor that has been doped, that is, into which a doping agent has been introduced, giving it different electrical properties than the intrinsic semiconductor....
. A semiconductor doped to such high levels that it acts more like a conductor than a semiconductor is referred to as degenerate
Degenerate semiconductor
A degenerate semiconductor is a semiconductor with such a high level of doping that the material starts to act more like a metal than as a semiconductor....
.
In the context of phosphor
Phosphor
A phosphor, most generally, is a substance that exhibits the phenomenon of luminescence. Somewhat confusingly, this includes both phosphorescent materials, which show a slow decay in brightness , and fluorescent materials, where the emission decay takes place over tens of nanoseconds...
s and scintillator
Scintillator
A scintillator is a special material, which exhibits scintillation—the property of luminescence when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate, i.e., reemit the absorbed energy in the form of light...
s, doping is better known as activation
Activator (phosphor)
In phosphors and scintillators, the activator is the element added as dopant to the crystal of the material to create desired type of nonhomogeneities....
.
History
Its effects were long known empirically in such devices as crystal radio detectors and selenium rectifiers. However, semiconductor doping was formally first developed by John Robert WoodyardJohn Robert Woodyard
John Robert Woodyard was a U.S. physicist who made important contributions to the technology of microwave electronics and invented "doping" to improve the performance of semiconductors.-Life:...
working at Sperry Gyroscope Company during World War II
World War II
World War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
. The demands of his work on radar
Radar
Radar is an object-detection system which uses radio waves to determine the range, altitude, direction, or speed of objects. It can be used to detect aircraft, ships, spacecraft, guided missiles, motor vehicles, weather formations, and terrain. The radar dish or antenna transmits pulses of radio...
denied Woodyard the opportunity to pursue research on semiconductor doping. However, after the war ended, his patent
Patent
A patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
proved the grounds of extensive litigation by Sperry Rand. Related work was performed at Bell Labs
Bell Labs
Bell Laboratories is the research and development subsidiary of the French-owned Alcatel-Lucent and previously of the American Telephone & Telegraph Company , half-owned through its Western Electric manufacturing subsidiary.Bell Laboratories operates its...
by Gordon K. Teal
Gordon K. Teal
Gordon Kidd Teal invented a method of applying the Czochralski method to produce extremely pure germanium single crystals used in making greatly improved transistors. He, together with Morgan Sparks invented a modification of the process that produced the configuration necessary for the...
and Morgan Sparks
Morgan Sparks
Morgan Sparks was an American scientist and engineer who helped develop the microwatt bipolar junction transistor in 1951, which was a critical step in making transistors usable for every-day electronics...
.
Process
Some dopantDopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
s are added as the (usually silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
) boule
Boule (crystal)
A boule is a single-crystal ingot produced by synthetic means. A boule of silicon is the starting material for most of the integrated circuits used today....
is grown, giving each wafer
Wafer (electronics)
A wafer is a thin slice of semiconductor material, such as a silicon crystal, used in the fabrication of integrated circuits and other microdevices...
an almost uniform initial doping. To define circuit elements, selected areas — typically controlled by photolithography
Photolithography
Photolithography is a process used in microfabrication to selectively remove parts of a thin film or the bulk of a substrate. It uses light to transfer a geometric pattern from a photomask to a light-sensitive chemical "photoresist", or simply "resist," on the substrate...
— are further doped by such processes as diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
and ion implantation
Ion implantation
Ion implantation is a materials engineering process by which ions of a material are accelerated in an electrical field and impacted into another solid. This process is used to change the physical, chemical, or electrical properties of the solid...
, the latter method being more popular in large production runs because of increased controllability.
Small numbers of dopant atoms can change the ability of a semiconductor to conduct electricity. When on the order of one dopant atom is added per 100 million atoms, the doping is said to be low or light. When many more dopant atoms are added, on the order of one per ten thousand atoms, the doping is referred to as heavy or high. This is often shown as n+ for n-type
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
doping or p+ for p-type
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
doping. (See the article on semiconductors for a more detailed description of the doping mechanism.)
Group IV semiconductors
(Note: When discussing periodic table groups, semiconductor physicists always use an older notation, not the current IUPAC group notation. For example, the carbon groupCarbon group
The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
is called "Group IV", not "Group 14".)
For the Group IV
Carbon group
The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
semiconductors such as silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, and silicon carbide
Silicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
, the most common dopants are acceptors
Acceptor (semiconductors)
In semiconductor physics, an acceptor is a dopant atom that when added to a semiconductor can form p-type regions.For example, when silicon , having four valence electrons, needs to be doped as a p-type semiconductor, elements from group III like boron or aluminium , having three valence...
from Group III
Boron group
The boron group is the series of elements in group 13 of the periodic table, comprising boron , aluminium , gallium , indium , thallium , and ununtrium . The elements in the boron group are characterized by having three electrons in their outer energy levels...
or donors
Donor (semiconductors)
In semiconductor physics, a donor is a dopant atom that, when added to a semiconductor, can form n-type regions.For example, when silicon , having four valence electrons, needs to be doped as an n-type semiconductor, elements from group V like phosphorus or arsenic can be used because they have...
from Group V
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
elements. Boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, and occasionally gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
are used to dope silicon. Boron is the p-type dopant
Acceptor (semiconductors)
In semiconductor physics, an acceptor is a dopant atom that when added to a semiconductor can form p-type regions.For example, when silicon , having four valence electrons, needs to be doped as a p-type semiconductor, elements from group III like boron or aluminium , having three valence...
of choice for silicon integrated circuit production because it diffuses at a rate that makes junction depths easily controllable. Phosphorus is typically used for bulk-doping of silicon wafers, while arsenic is used to diffuse junctions, because it diffuses more slowly than phosphorus and is thus more controllable.
By doping pure silicon with Group V
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
elements such as phosphorus, extra valence electrons are added that become unbonded from individual atoms and allow the compound to be an electrically conductive n-type semiconductor
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
. Doping with Group III
Boron group
The boron group is the series of elements in group 13 of the periodic table, comprising boron , aluminium , gallium , indium , thallium , and ununtrium . The elements in the boron group are characterized by having three electrons in their outer energy levels...
elements, which are missing the fourth valence electron, creates "broken bonds" (holes) in the silicon lattice that are free to move. The result is an electrically conductive p-type semiconductor
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
. In this context, a Group V
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
element is said to behave as an electron donor, and a group III
Boron group
The boron group is the series of elements in group 13 of the periodic table, comprising boron , aluminium , gallium , indium , thallium , and ununtrium . The elements in the boron group are characterized by having three electrons in their outer energy levels...
element as an acceptor. This is a key concept in the physics of a diode
Diode
In electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
.
Very heavily doped semiconductor behaves more like a good conductor (metal) and thus exhibits more linear positive thermal coefficient. Such effect is used for instance in sensistor
Sensistor
Sensistor is a resistor whose resistance changes with temperature.The resistance increases exponentially with temperature, that is the temperature coefficient is positive Sensistor is a resistor whose resistance changes with temperature.The resistance increases exponentially with temperature, that...
s. Lower dosage of doping is used in other types (NTC or PTC) thermistor
Thermistor
A thermistor is a type of resistor whose resistance varies significantly with temperature, more so than in standard resistors. The word is a portmanteau of thermal and resistor...
s.
Compensation
In most cases many types of impurities will be present in the resultant doped semiconductor. If an equal number of donors and acceptors are present in the semiconductor, the extra core electrons provided by the former will be used to satisfy the broken bonds due to the latter, so that doping produces no free carriers of either type. This phenomenon is known as compensation, and occurs at the p-n junctionP-n junction
A p–n junction is formed at the boundary between a P-type and N-type semiconductor created in a single crystal of semiconductor by doping, for example by ion implantation, diffusion of dopants, or by epitaxy .If two separate pieces of material were used, this would...
in the vast majority of semiconductor devices. Partial compensation, where donors outnumber acceptors or vice versa, allows device makers to repeatedly reverse the type of a given portion of the material by applying successively higher doses of dopants.
Although compensation can be used to increase or decrease the number of donors or acceptors, the electron and hole mobility
Electron mobility
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor, when pulled by an electric field. In semiconductors, there is an analogous quantity for holes, called hole mobility...
is always decreased by compensation because mobility is affected by the sum of the donor and acceptor ions.
Doping in organic conductors
Conductive polymerConductive polymer
Conductive polymers or, more precisely, intrinsically conducting polymers are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive...
s can be doped by adding chemical reactants to oxidize, or sometimes reduce, the system so that electrons are pushed into the conducting orbitals
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
within the already potentially conducting system. There are two primary methods of doping a conductive polymer, both of which use an oxidation-reduction (i.e., redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
) process.
- Chemical doping involves exposing a polymer such as melaninMelaninMelanin is a pigment that is ubiquitous in nature, being found in most organisms . In animals melanin pigments are derivatives of the amino acid tyrosine. The most common form of biological melanin is eumelanin, a brown-black polymer of dihydroxyindole carboxylic acids, and their reduced forms...
, typically a thin filmThin filmA thin film is a layer of material ranging from fractions of a nanometer to several micrometers in thickness. Electronic semiconductor devices and optical coatings are the main applications benefiting from thin film construction....
, to an oxidant such as iodineIodineIodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
or bromineBromineBromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
. Alternatively, the polymer can be exposed to a reductantReducing agentA reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
; this method is far less common, and typically involves alkali metals. - Electrochemical doping involves suspending a polymer-coated, working electrodeElectrodeAn electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
in an electrolyteElectrolyteIn chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
solution in which the polymer is insoluble along with separate counter and reference electrodes. An electric potential difference is created between the electrodes that causes a charge and the appropriate counter ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
from the electrolyteElectrolyteIn chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
to enter the polymer in the form of electron addition (i.e., n-doping) or removal (i.e., p-doping).
N-doping is much less common because the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
is oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
-rich, thus creating an oxidizing environment. An electron-rich, n-doped polymer will react immediately with elemental oxygen to de-dope (i.e., reoxidize to the neutral state) the polymer. Thus, chemical n-doping must be performed in an environment of inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
(e.g., argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
). Electrochemical n-doping is far more common in research, because it is easier to exclude oxygen from a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
in a sealed flask
Laboratory flask
Laboratory flasks are vessels which fall into the category of laboratory equipment known as glassware. In laboratory and other scientific settings, they are usually referred to simply as flasks...
. However, it is unlikely that n-doped conductive polymers are available commercially.
Magnetic doping
Research on magnetic doping has shown that considerable alteration of certain properties such as specific heat may be affected by small concentrations of an impurity; for example, dopant impurities in semiconducting ferromagnetic alloys can generate different properties as first predicted by White, Hogan, Suhl and Nakamura.Single dopants in semiconductors
The sensitive dependence of a semiconductor’s electronic, optical, and magnetic properties on dopants has provided an extensive range of tunable phenomena to explore and apply to devices. Recently it has become possible to move past the tunable properties of an ensemble of dopants and to identify the effects of a solitary dopant on commercial device performance as well as locally on the fundamental properties of a semiconductor. New applications have become available that require the discrete character of a single dopant, such as single-spin devices in the area of quantum information or single-dopant transistors. Dramatic advances in the past decade towards observing, controllably creating and manipulating single dopants, as well as their application in novel devices have allowed opening the new field of solotronics (solitary dopant optoelectronics).Neutron transmutation doping
NeutronNeutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
transmutation
Nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'...
doping (NTD) is an unusual doping method for special applications. Most commonly, it is used to dope silicon n-type in high-power electronics. It is based on the conversion of the Si-30 isotope into phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
atom by neutron absorption as follows:
-
-
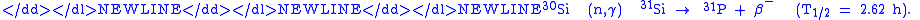
In practice, the silicon is typically placed near a nuclear reactorNuclear reactorA nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
to receive the neutrons. As neutrons continue to pass through the silicon, more and more phosphorus atoms are produced by transmutation, and therefore the doping becomes more and more strongly n-type. NTD is a far less common doping method than diffusion or ion implantation, but it has the advantage of creating an extremely uniform dopant distribution.
See also
- Extrinsic semiconductorExtrinsic semiconductorAn extrinsic semiconductor is a semiconductor that has been doped, that is, into which a doping agent has been introduced, giving it different electrical properties than the intrinsic semiconductor....
- Intrinsic semiconductorIntrinsic semiconductorAn intrinsic semiconductor, also called an undoped semiconductor or i-type semiconductor, is a pure semiconductor without any significant dopant species present. The number of charge carriers is therefore determined by the properties of the material itself instead of the amount of impurities...
- p-n junctionP-n junctionA p–n junction is formed at the boundary between a P-type and N-type semiconductor created in a single crystal of semiconductor by doping, for example by ion implantation, diffusion of dopants, or by epitaxy .If two separate pieces of material were used, this would...
- List of semiconductor materials
- Extrinsic semiconductor
-

