.gif)
Entropy (thermodynamic views)
Encyclopedia
In thermodynamics
, entropy is a measure of how much of the energy of a system
is potentially available to do work and how much of it is potentially manifest as heat
. In classical thermodynamics, the entropy is defined only for a system in thermodynamic equilibrium
. A thermodynamic system is any physical object or region of space that can be described by its thermodynamic quantities such as temperature
, pressure
, volume
and density
. In simple terms, the second law of thermodynamics
states that for a system, the intensive thermodynamic quantities such as temperature
, pressure
, and chemical potential
tend to become more uniform as time goes by, unless there is an outside influence which works to maintain the differences.
There are two related definitions of entropy. The first definition is the thermodynamic definition. It was developed by Rudolf Clausius
and essentially describes how to measure the entropy of an isolated system in thermodynamic equilibrium
. It makes no reference to the microscopic nature of matter. The second definition is the statistical
definition developed by Ludwig Boltzmann
in the 1870s. This definition describes the entropy as a measure of the number of possible microscopic configurations of the individual atoms and molecules of the system (microstates) which would give rise to the observed macroscopic state (macrostate) of the system. Boltzmann then went on to show that this definition of entropy was equal to the thermodynamic entropy to within a constant number which has since been known as Boltzmann's constant.
This article is concerned with the thermodynamic definition of entropy. Although thermodynamic entropy is a self-contained subject, it should be understood in parallel with the statistical definition. When the thermodynamic definition becomes most difficult to understand, the statistical definition brings a simple explanation, and where the link between the statistical theory and experiment becomes extended, the thermodynamic theory delivers a straightforward answer.
Clausius defined the change in entropy of a thermodynamic system, during a reversible process
of a thermodynamic system, during a reversible process
, as
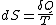
where
Note that the small amount δQ of energy transferred by heating is denoted by δQ rather than dQ, because Q is not a state function
while the entropy is.
Clausius gave the quantity S the name "entropy", from the Greek word τρoπή, "transformation". Since this definition involves only differences in entropy, the entropy itself is only defined up to
an arbitrary additive constant.
When a process is irreversible, the above definition must be replaced by the statement that the entropy change is equal to the amount of energy required to return the system to its original state by a reversible transformation at a constant temperature, divided by that temperature. This is explained in more detail below.
, modeled as a universe consisting of surroundings and systems and made up of quantities of matter, pressure differences, density differences, and temperature differences all tend to equalize over time. In the example of ice melting, the difference in temperature between a warm room, which is the surroundings, and a cold glass of water and ice, which is the system, is equalized as heat from the warm surroundings are transferred to the cooler ice and water mixture.
 Over time the temperature of the glass and its contents and the temperature of the room achieve balance. The entropy of the room has decreased. However, as calculated in the example, the entropy of the system of ice and water has increased more than the entropy of the surrounding room has decreased. In an isolated system
Over time the temperature of the glass and its contents and the temperature of the room achieve balance. The entropy of the room has decreased. However, as calculated in the example, the entropy of the system of ice and water has increased more than the entropy of the surrounding room has decreased. In an isolated system
such as the room and ice water taken together, the dispersal of energy from warmer to cooler regions always results in a net increase in entropy. Thus, when the universe of the room and ice water system has reached a temperature equilibrium, the entropy change from the initial state is at a maximum. The entropy of the thermodynamic system
is a measure of how far the equalization has progressed.
A special case of entropy increase, the entropy of mixing
, occurs when two or more different substances are mixed. If the substances are at the same temperature and pressure, there will be no net exchange of heat or work - the entropy increase will be entirely due to the mixing of the different substances.
From a macroscopic perspective, in classical thermodynamics the entropy is interpreted simply as a state function
of a thermodynamic system
: that is, a property depending only on the current state of the system, independent of how that state came to be achieved. The state function has the important property that, when multiplied by a reference temperature, it can be understood as a measure of the amount of energy
in a physical system that cannot be used to do thermodynamic work
; i.e., work mediated by thermal energy. More precisely, in any process where the system gives up energy ΔE, and its entropy falls by ΔS, a quantity at least TR ΔS of that energy must be given up to the system's surroundings as unusable heat
(TR is the temperature of the system's external surroundings). Otherwise the process will not go forward.
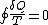
That means the line integral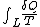 is path independent.
is path independent.
So we can define a state function S called entropy, which satisfied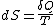
With this we can only obtain the difference of entropy by integrating the above formula. To obtain the absolute value, we need Third Law of Thermodynamics
, which states that S=0 at absolute zero
for perfect crystals.
Now reverse the reversible process and combine it with the said irreversible process. Applying Clausius inequality on this loop,

Thus,
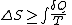
where the equality holds if the transformation is reversible.
Notice that if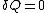 , then ΔS ≥ 0. This is the Second Law of Thermodynamics
, then ΔS ≥ 0. This is the Second Law of Thermodynamics
.
Suppose a system is thermally and mechanically isolated from the environment. For example, consider an insulating rigid box divided by a movable partition into two volumes, each filled with gas. If the pressure of one gas is higher, it will expand by moving the partition, thus performing work on the other gas. Also, if the gases are at different temperatures, heat can flow from one gas to the other provided the partition is an imperfect insulator. Our above result indicates that the entropy of the system as a whole will increase during these process (it could in principle remain constant, but this is unlikely.) Typically, there exists a maximum amount of entropy the system may possess under the circumstances. This entropy corresponds to a state of stable equilibrium, since a transformation to any other equilibrium state would cause the entropy to decrease, which is forbidden. Once the system reaches this maximum-entropy state, no part of the system can perform work on any other part. It is in this sense that entropy is a measure of the energy in a system that cannot be used to do work.
An irreversible process degrades the performance of a thermodynamic system designed to do work and results in entropy generation. Total entropy generation during a reversible process is zero. Thus entropy is a measure of the irreversibility and may be used to compare engineering processes and machines.
 Clausius' identification of S as a significant quantity was motivated by the study of reversible and irreversible thermodynamic transformations. A thermodynamic transformation is a change in a system's thermodynamic properties, such as temperature
Clausius' identification of S as a significant quantity was motivated by the study of reversible and irreversible thermodynamic transformations. A thermodynamic transformation is a change in a system's thermodynamic properties, such as temperature
and volume
. A transformation is reversible if it is a quasistatic process
which means that it is infinitesimal
ly close to thermodynamic equilibrium
at all times. Otherwise, the transformation is irreversible
. To illustrate this, consider a gas enclosed in a piston
chamber, whose volume may be changed by moving the piston. If we move the piston slowly enough, the density of the gas is always homogeneous, so the transformation is reversible. If we move the piston quickly, pressure waves are created, so the gas is not in equilibrium, and the transformation is irreversible.
A heat engine
is a thermodynamic system that can undergo a sequence of transformations which ultimately return it to its original state. Such a sequence is called a cyclic process, or simply a cycle. During some transformations, the engine may exchange energy with the environment. The net result of a cycle is (i) mechanical work
done by the system (which can be positive or negative
, the latter meaning that work is done on the engine), and (ii) heat energy transferred from one part of the environment to another. By the conservation of energy
, the net energy lost by the environment is equal to the work done by the engine.
If every transformation in the cycle is reversible, the cycle is reversible, and it can be run in reverse, so that the energy transfers occur in the opposite direction and the amount of work done switches sign.
For the case of a heat engine working between two temperatures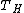 and
and 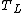 . The work produced by the engine on a cycle is given by the First law of thermodynamics
. The work produced by the engine on a cycle is given by the First law of thermodynamics
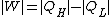
with the entropy production in the universe during the same time is given by the reduction of entropy in the hot source and the increase in the cold sink
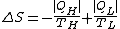
Combining these two relations
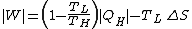
The first is the maximum possible work for a heat engine, given by a reversible engine, as one operating along a Carnot cycle
. Finally

This equation tells us that the production of work is reduced by the generation of entropy, being the term the lost work by the machine.
the lost work by the machine.
Correspondingly, the amount of heat discarded to the cold sink is increased by the entropy generation
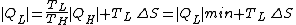
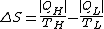
and the work to extract heat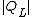 from the cold source is
from the cold source is
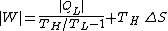
The first term is the minimum required work, which corresponds to a reversible refrigerator, so we have
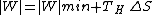
i.e., the refrigerator compressor has to perform extra work to compensate for the energy waste due to entropy production.
s, it is quite difficult to measure
the entropy of a system. The techniques for doing so are based on the thermodynamic definition of the entropy, and require extremely careful calorimetry
.
For simplicity, we will examine a mechanical system, whose thermodynamic state may be specified by its volume V and pressure P. In order to measure the entropy of a specific state, we must first measure the heat capacity
at constant volume and at constant pressure (denoted CV and CP respectively), for a successive set of states intermediate between a reference state and the desired state. The heat capacities are related to the entropy S and the temperature T by
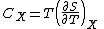
where the X subscript refers to either constant volume or constant pressure. This may be integrated numerically
to obtain a change in entropy:
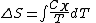
We can thus obtain the entropy of any state (P,V) with respect to a reference state (P0,V0). The exact formula depends on our choice of intermediate states. For example, if the reference state has the same pressure as the final state,

In addition, if the path between the reference and final states lies across any first order phase transition
, the latent heat
associated with the transition must be taken into account.
The entropy of the reference state must be determined independently. Ideally, one chooses a reference state at an extremely high temperature, at which the system exists as a gas. The entropy in such a state would be that of a classical ideal gas plus contributions from molecular rotations and vibrations, which may be determined spectroscopically
. Choosing a low temperature reference state is sometimes problematic since the entropy at low temperatures may behave in unexpected ways. For instance, a calculation of the entropy of ice
by the latter method, assuming no entropy at zero temperature, falls short of the value obtained with a high-temperature reference state by 3.41 J/(mol·K). This is due to the "zero-point" entropy of ice mentioned earlier.
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, entropy is a measure of how much of the energy of a system
System
System is a set of interacting or interdependent components forming an integrated whole....
is potentially available to do work and how much of it is potentially manifest as heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
. In classical thermodynamics, the entropy is defined only for a system in thermodynamic equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
. A thermodynamic system is any physical object or region of space that can be described by its thermodynamic quantities such as temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
and density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
. In simple terms, the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
states that for a system, the intensive thermodynamic quantities such as temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, and chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
tend to become more uniform as time goes by, unless there is an outside influence which works to maintain the differences.
There are two related definitions of entropy. The first definition is the thermodynamic definition. It was developed by Rudolf Clausius
Rudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
and essentially describes how to measure the entropy of an isolated system in thermodynamic equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
. It makes no reference to the microscopic nature of matter. The second definition is the statistical
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
definition developed by Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
in the 1870s. This definition describes the entropy as a measure of the number of possible microscopic configurations of the individual atoms and molecules of the system (microstates) which would give rise to the observed macroscopic state (macrostate) of the system. Boltzmann then went on to show that this definition of entropy was equal to the thermodynamic entropy to within a constant number which has since been known as Boltzmann's constant.
This article is concerned with the thermodynamic definition of entropy. Although thermodynamic entropy is a self-contained subject, it should be understood in parallel with the statistical definition. When the thermodynamic definition becomes most difficult to understand, the statistical definition brings a simple explanation, and where the link between the statistical theory and experiment becomes extended, the thermodynamic theory delivers a straightforward answer.
Clausius defined the change in entropy
 of a thermodynamic system, during a reversible process
of a thermodynamic system, during a reversible processReversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
, as
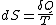
where
- δQ is a small amount of heatHeatIn physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
introduced reversibly to the system, - T is a constant absolute temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
Note that the small amount δQ of energy transferred by heating is denoted by δQ rather than dQ, because Q is not a state function
State function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
while the entropy is.
Clausius gave the quantity S the name "entropy", from the Greek word τρoπή, "transformation". Since this definition involves only differences in entropy, the entropy itself is only defined up to
Up to
In mathematics, the phrase "up to x" means "disregarding a possible difference in x".For instance, when calculating an indefinite integral, one could say that the solution is f "up to addition by a constant," meaning it differs from f, if at all, only by some constant.It indicates that...
an arbitrary additive constant.
When a process is irreversible, the above definition must be replaced by the statement that the entropy change is equal to the amount of energy required to return the system to its original state by a reversible transformation at a constant temperature, divided by that temperature. This is explained in more detail below.
Introduction
In a thermodynamic systemThermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
, modeled as a universe consisting of surroundings and systems and made up of quantities of matter, pressure differences, density differences, and temperature differences all tend to equalize over time. In the example of ice melting, the difference in temperature between a warm room, which is the surroundings, and a cold glass of water and ice, which is the system, is equalized as heat from the warm surroundings are transferred to the cooler ice and water mixture.

Isolated system
In the natural sciences an isolated system, as contrasted with an open system, is a physical system without any external exchange. If it has any surroundings, it does not interact with them. It obeys in particular the first of the conservation laws: its total energy - mass stays constant...
such as the room and ice water taken together, the dispersal of energy from warmer to cooler regions always results in a net increase in entropy. Thus, when the universe of the room and ice water system has reached a temperature equilibrium, the entropy change from the initial state is at a maximum. The entropy of the thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
is a measure of how far the equalization has progressed.
A special case of entropy increase, the entropy of mixing
Entropy of mixing
In thermodynamics the entropy of mixing is the increase in the total entropy of a compound system, when different and chemically non-reacting chemical substances or material components are mixed by removing partition between the system's initially separate volumes...
, occurs when two or more different substances are mixed. If the substances are at the same temperature and pressure, there will be no net exchange of heat or work - the entropy increase will be entirely due to the mixing of the different substances.
From a macroscopic perspective, in classical thermodynamics the entropy is interpreted simply as a state function
State function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
: that is, a property depending only on the current state of the system, independent of how that state came to be achieved. The state function has the important property that, when multiplied by a reference temperature, it can be understood as a measure of the amount of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
in a physical system that cannot be used to do thermodynamic work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
; i.e., work mediated by thermal energy. More precisely, in any process where the system gives up energy ΔE, and its entropy falls by ΔS, a quantity at least TR ΔS of that energy must be given up to the system's surroundings as unusable heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
(TR is the temperature of the system's external surroundings). Otherwise the process will not go forward.
Definition
According to the Clausius equality, for a reversible process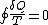
That means the line integral
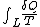 is path independent.
is path independent.So we can define a state function S called entropy, which satisfied
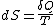
With this we can only obtain the difference of entropy by integrating the above formula. To obtain the absolute value, we need Third Law of Thermodynamics
Third law of thermodynamics
The third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
, which states that S=0 at absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
for perfect crystals.
Entropy change in irreversible transformations
We now consider irreversible transformations. For any irreversible process, since entropy is a state function, we can always connect the initial and terminal status with an imaginary reversible process and integrating on that path to calculate the difference in entropy.Now reverse the reversible process and combine it with the said irreversible process. Applying Clausius inequality on this loop,

Thus,
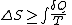
where the equality holds if the transformation is reversible.
Notice that if
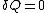 , then ΔS ≥ 0. This is the Second Law of Thermodynamics
, then ΔS ≥ 0. This is the Second Law of ThermodynamicsSecond law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
.
Suppose a system is thermally and mechanically isolated from the environment. For example, consider an insulating rigid box divided by a movable partition into two volumes, each filled with gas. If the pressure of one gas is higher, it will expand by moving the partition, thus performing work on the other gas. Also, if the gases are at different temperatures, heat can flow from one gas to the other provided the partition is an imperfect insulator. Our above result indicates that the entropy of the system as a whole will increase during these process (it could in principle remain constant, but this is unlikely.) Typically, there exists a maximum amount of entropy the system may possess under the circumstances. This entropy corresponds to a state of stable equilibrium, since a transformation to any other equilibrium state would cause the entropy to decrease, which is forbidden. Once the system reaches this maximum-entropy state, no part of the system can perform work on any other part. It is in this sense that entropy is a measure of the energy in a system that cannot be used to do work.
An irreversible process degrades the performance of a thermodynamic system designed to do work and results in entropy generation. Total entropy generation during a reversible process is zero. Thus entropy is a measure of the irreversibility and may be used to compare engineering processes and machines.
Heat engines

Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
. A transformation is reversible if it is a quasistatic process
Quasistatic process
In thermodynamics, a quasistatic process is a thermodynamic process that happens infinitely slowly. However, it is very important of note that no real process is quasistatic...
which means that it is infinitesimal
Infinitesimal
Infinitesimals have been used to express the idea of objects so small that there is no way to see them or to measure them. The word infinitesimal comes from a 17th century Modern Latin coinage infinitesimus, which originally referred to the "infinite-th" item in a series.In common speech, an...
ly close to thermodynamic equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
at all times. Otherwise, the transformation is irreversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
. To illustrate this, consider a gas enclosed in a piston
Piston
A piston is a component of reciprocating engines, reciprocating pumps, gas compressors and pneumatic cylinders, among other similar mechanisms. It is the moving component that is contained by a cylinder and is made gas-tight by piston rings. In an engine, its purpose is to transfer force from...
chamber, whose volume may be changed by moving the piston. If we move the piston slowly enough, the density of the gas is always homogeneous, so the transformation is reversible. If we move the piston quickly, pressure waves are created, so the gas is not in equilibrium, and the transformation is irreversible.
A heat engine
Heat engine
In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy that brings the working substance...
is a thermodynamic system that can undergo a sequence of transformations which ultimately return it to its original state. Such a sequence is called a cyclic process, or simply a cycle. During some transformations, the engine may exchange energy with the environment. The net result of a cycle is (i) mechanical work
Mechanical work
In physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
done by the system (which can be positive or negative
Sign (mathematics)
In mathematics, the word sign refers to the property of being positive or negative. Every nonzero real number is either positive or negative, and therefore has a sign. Zero itself is signless, although in some contexts it makes sense to consider a signed zero...
, the latter meaning that work is done on the engine), and (ii) heat energy transferred from one part of the environment to another. By the conservation of energy
Conservation of energy
The nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
, the net energy lost by the environment is equal to the work done by the engine.
If every transformation in the cycle is reversible, the cycle is reversible, and it can be run in reverse, so that the energy transfers occur in the opposite direction and the amount of work done switches sign.
For the case of a heat engine working between two temperatures
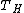 and
and 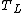 . The work produced by the engine on a cycle is given by the First law of thermodynamics
. The work produced by the engine on a cycle is given by the First law of thermodynamicsFirst law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
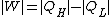
with the entropy production in the universe during the same time is given by the reduction of entropy in the hot source and the increase in the cold sink
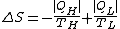
Combining these two relations
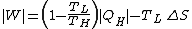
The first is the maximum possible work for a heat engine, given by a reversible engine, as one operating along a Carnot cycle
Carnot cycle
The Carnot cycle is a theoretical thermodynamic cycle proposed by Nicolas Léonard Sadi Carnot in 1824 and expanded by Benoit Paul Émile Clapeyron in the 1830s and 40s. It can be shown that it is the most efficient cycle for converting a given amount of thermal energy into work, or conversely,...
. Finally

This equation tells us that the production of work is reduced by the generation of entropy, being the term
 the lost work by the machine.
the lost work by the machine.Correspondingly, the amount of heat discarded to the cold sink is increased by the entropy generation
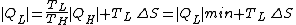
Refrigerators
The same principle can be applied to a refrigerator. In this case the entropy production is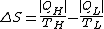
and the work to extract heat
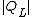 from the cold source is
from the cold source is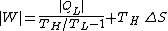
The first term is the minimum required work, which corresponds to a reversible refrigerator, so we have
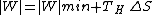
i.e., the refrigerator compressor has to perform extra work to compensate for the energy waste due to entropy production.
Entropy measurement
In thermal experimentExperiment
An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the validity of a hypothesis. Experiments vary greatly in their goal and scale, but always rely on repeatable procedure and logical analysis of the results...
s, it is quite difficult to measure
Measurement
Measurement is the process or the result of determining the ratio of a physical quantity, such as a length, time, temperature etc., to a unit of measurement, such as the metre, second or degree Celsius...
the entropy of a system. The techniques for doing so are based on the thermodynamic definition of the entropy, and require extremely careful calorimetry
Calorimetry
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
.
For simplicity, we will examine a mechanical system, whose thermodynamic state may be specified by its volume V and pressure P. In order to measure the entropy of a specific state, we must first measure the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
at constant volume and at constant pressure (denoted CV and CP respectively), for a successive set of states intermediate between a reference state and the desired state. The heat capacities are related to the entropy S and the temperature T by
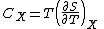
where the X subscript refers to either constant volume or constant pressure. This may be integrated numerically
Numerical integration
In numerical analysis, numerical integration constitutes a broad family of algorithms for calculating the numerical value of a definite integral, and by extension, the term is also sometimes used to describe the numerical solution of differential equations. This article focuses on calculation of...
to obtain a change in entropy:
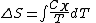
We can thus obtain the entropy of any state (P,V) with respect to a reference state (P0,V0). The exact formula depends on our choice of intermediate states. For example, if the reference state has the same pressure as the final state,

In addition, if the path between the reference and final states lies across any first order phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
, the latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
associated with the transition must be taken into account.
The entropy of the reference state must be determined independently. Ideally, one chooses a reference state at an extremely high temperature, at which the system exists as a gas. The entropy in such a state would be that of a classical ideal gas plus contributions from molecular rotations and vibrations, which may be determined spectroscopically
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
. Choosing a low temperature reference state is sometimes problematic since the entropy at low temperatures may behave in unexpected ways. For instance, a calculation of the entropy of ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
by the latter method, assuming no entropy at zero temperature, falls short of the value obtained with a high-temperature reference state by 3.41 J/(mol·K). This is due to the "zero-point" entropy of ice mentioned earlier.
See also
- EntropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
- EnthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
- Fundamental thermodynamic relation
- Thermodynamic free energyThermodynamic free energyThe thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
- History of entropyHistory of entropyThe concept of entropy developed in response to the observation that a certain amount of functional energy released from combustion reactions is always lost to dissipation or friction and is thus not transformed into useful work...
- Entropy (statistical views)Entropy (statistical views)In classical statistical mechanics, the entropy function earlier introduced by Clausius is changed to statistical entropy using probability theory...
Further reading
- Goldstein, Martin, and Inge F., 1993. The Refrigerator and the Universe. Harvard Univ. Press. A gentle introduction at a lower level than this entry.

