
Endoplasmic reticulum
Encyclopedia
Organelle
In cell biology, an organelle is a specialized subunit within a cell that has a specific function, and is usually separately enclosed within its own lipid bilayer....
of cells
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
in eukaryotic organisms
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
that forms an interconnected network of tubules, vesicles
Vesicle (biology)
A vesicle is a bubble of liquid within another liquid, a supramolecular assembly made up of many different molecules. More technically, a vesicle is a small membrane-enclosed sack that can store or transport substances. Vesicles can form naturally because of the properties of lipid membranes , or...
, and cisterna
Cisterna
A cisterna comprises a flattened membrane disk that makes up the Golgi apparatus. A typical Golgi has anywhere from 3 to 7 cisternae stacked upon each other like a stack of dinner plates, but there are usually around 6...
e. Rough endoplasmic reticula synthesize proteins, while smooth endoplasmic reticula synthesize lipids and steroids, metabolize carbohydrates and steroids (but not lipids), and regulate calcium concentration, drug metabolism
Drug metabolism
Drug metabolism is the biochemical modification of pharmaceutical substances by living organisms, usually through specialized enzymatic systems. This is a form of xenobiotic metabolism. Drug metabolism often converts lipophilic chemical compounds into more readily excreted polar products...
, and attachment of receptors on cell membrane proteins. Sarcoplasmic reticula solely regulate calcium levels.
The lacey membranes of the endoplasmic reticulum were first seen by Keith R. Porter
Keith R. Porter
Keith Roberts Porter was a Canadian cell biologist. He did pioneering biology research using electron microscopy of cells , such as work on the 9 + 2 microtubule structure in the axoneme of cilia. Porter also contributed to the development of other experimental methods for cell culture and nuclear...
, Albert Claude
Albert Claude
Albert Claude was a Belgian biologist who shared the Nobel Prize in Physiology or Medicine in 1974 with Christian de Duve and George Emil Palade. He studied engineering, and then medicine...
, and Ernest F. Fullam in 1945.
Structure
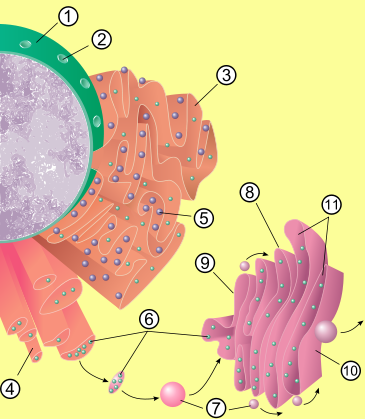
Cisterna
A cisterna comprises a flattened membrane disk that makes up the Golgi apparatus. A typical Golgi has anywhere from 3 to 7 cisternae stacked upon each other like a stack of dinner plates, but there are usually around 6...
e (sac-like structures) held together by the cytoskeleton
Cytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
. The phospholipid membrane encloses a space, the cisternal space (or lumen), which is continuous with the perinuclear space but separate from the cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
. The functions of the endoplasmic reticulum vary greatly depending on the exact type of endoplasmic reticulum and the type of cell in which it resides. The three varieties are called rough endoplasmic reticulum, smooth endoplasmic reticulum, and sarcoplasmic reticulum.
The quantity of RER and SER in a cell can quickly interchange from one type to the other, depending on changing metabolic needs: One type will undergo numerous changes including new proteins embedded in the membranes in order to transform. Also, massive changes in the protein content can occur without any noticeable structural changes, depending on the enzymatic needs of the cell (as per the functions listed below).
Rough endoplasmic reticulum
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
s giving it a "rough" appearance (hence its name). The binding site of the Ribosome on RER is a Glycoprotein receptor called Ribophorin
Ribophorin
Ribophorins are ribosome receptor proteins that aids in the binding of ribosomes to the rough endoplasmic reticulum . It is an integral protein within the rough endoplasmic reticular membrane. As it is directly related with protein translation, ribophorins are not found in smooth endoplasmic...
. However, the ribosomes bound to the RER at any one time are not a stable part of this organelle's structure as ribosomes are constantly being bound and released from the membrane. A ribosome binds to the ER only when it begins to synthesize a protein destined for the secretory pathway
Secretory pathway
The secretory pathway is a series of steps a cell uses to move proteins out of the cell; a process known as secretion. The path of a protein destined for secretion has its origins in the rough endoplasmic reticulum, a membrane-bound compartment in the cell...
. Here, a ribosome in the cytosol begins synthesizing a protein until a signal recognition particle
Signal recognition particle
The signal recognition particle is an abundant, cytosolic, universally conserved ribonucleoprotein that recognizes and targets specific proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes....
recognizes the pre-piece of 5-15 hydrophobic amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s preceded by a positively charged amino acid. This signal sequence allows the recognition particle to bind to the ribosome, causing the ribosome to bind to the RER and pass the new protein through the ER membrane. The pre-piece is then cleaved off within the lumen of the ER and the ribosome released back into the cytosol.
The membrane of the RER is continuous with the outer layer of the nuclear envelope
Nuclear envelope
A nuclear envelope is a double lipid bilayer that encloses the genetic material in eukaryotic cells. The nuclear envelope also serves as the physical barrier, separating the contents of the nucleus from the cytosol...
. Although there is no continuous membrane between the RER and the Golgi apparatus
Golgi apparatus
The Golgi apparatus is an organelle found in most eukaryotic cells. It was identified in 1898 by the Italian physician Camillo Golgi, after whom the Golgi apparatus is named....
, membrane-bound vesicles shuttle proteins between these two compartments. Vesicles are surrounded by coating proteins called COPI and COPII. COPII
COPII
COPII is a type of vesicle coat protein that transports proteins from the rough endoplasmic reticulum to the Golgi apparatus. This is termed anterograde transport. The name "COPII" refers to the specific coat protein complex that initiates the budding process...
targets vesicles to the golgi and COPI
COPI
COPI is a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum , where they were originally synthesized and between golgi compartments. This type of transport is termed as retrograde transport...
marks them to be brought back to the RER. The RER works in concert with the Golgi complex to target new proteins
Protein targeting
Protein targeting or protein sorting is the mechanism by which a cell transports proteins to the appropriate positions in the cell or outside of it. Sorting targets can be the inner space of an organelle, any of several interior membranes, the cell's outer membrane, or its exterior via secretion...
to their proper destinations. A second method of transport out of the ER is areas called membrane contact site
Membrane contact site
Membrane contact sites are areas within a cell where the membranes of two organelles are arranged in close proximity . These sites are thought to be important in the transport of small molecules such as lipids between organelles, and offer an alternative to the classical mechanism of lipid...
s, where the membranes of the ER and other organelles are held closely together, allowing the transfer of lipids and other small molecules.
The RER is key in multiple functions:
- LysosomalLysosomethumb|350px|Schematic of typical animal cell, showing subcellular components. [[Organelle]]s: [[nucleoli]] [[cell nucleus|nucleus]] [[ribosomes]] [[vesicle |vesicle]] rough [[endoplasmic reticulum]]...
enzymes with a mannose-6-phosphateMannose-6-phosphateMannose-6-phosphate is a molecule bound by lectin in the immune system. M6P is converted to fructose 6-phosphate by mannose phosphate isomerase....
marker added in the cis-Golgi network - SecretedSecretionSecretion is the process of elaborating, releasing, and oozing chemicals, or a secreted chemical substance from a cell or gland. In contrast to excretion, the substance may have a certain function, rather than being a waste product...
proteins, either secreted constitutively with no tag or secreted in a regulatory manner involving clathrinClathrinClathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1975. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice...
and paired basic amino acids in the signal peptideSignal peptideA signal peptide is a short peptide chain that directs the transport of a protein.Signal peptides may also be called targeting signals, signal sequences, transit peptides, or localization signals....
. - Integral membrane proteins that stay embedded in the membrane as vesicles exit and bind to new membranes. RabRab (G-protein)The Rab family of proteins is a member of the Ras superfamily of monomeric G proteins. Approximately 70 types of Rabs have now been identified in humans. Rab GTPases regulate many steps of membrane traffic, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane...
proteins are key in targeting the membrane; SNAPSNAP-25Synaptosomal-associated protein 25 is a protein that in humans is encoded by the SNAP25 gene. The SNAP-25 protein is a component of the SNARE complex, which is proposed to account for the specificity of membrane fusion and to directly execute fusion by forming a tight complex that brings the...
and SNARESnareSnare may refer to:* Snare trap, a kind of trap used for capturing animals* Snare drum* SNARE , a family of proteins involved in vesicle fusion* The Snares, a group of islands approximately 200 kilometres south of New Zealand...
proteins are key in the fusion event. - Initial glycosylationGlycosylationGlycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
as assembly continues. This is N-linked (O-linking occurs in the golgi).- N-linked glycosylation: If the protein is properly folded, glycosyltransferaseGlycosyltransferaseGlycosyltransferases are enzymes that act as a catalyst for the transfer of a monosaccharide unit from an activated nucleotide sugar to a glycosyl acceptor molecule, usually an alcohol....
recognizes the AA sequence NAsparagineAsparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
XSSerineSerine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
or NAsparagineAsparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
XTThreonineThreonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
(with the S/T residue phosphorylated) and adds a 14-sugar backbone (2-N-acetylglucosamine, 9-branching mannoseMannoseMannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
, and 3-glucoseGlucoseGlucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
at the end) to the side-chain nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
of Asn.
- N-linked glycosylation: If the protein is properly folded, glycosyltransferase
Endoplasmic Reticulum Stress
Disturbances in redox regulation, calcium regulation, glucose deprivation, and viral infection can lead to ER stress. It is a state in which the folding of proteins slows leading to an increase in unfolded proteins. This ER stress is emerging as a potential cause of damage in hypoxia/ischemia, insulin resistance and other disorders.Smooth endoplasmic reticulum
The smooth endoplasmic reticulum (SER) has functions in several metabolic processes, including synthesis of lipids and steroids, metabolism of carbohydrates, regulation of calcium concentration, drug detoxification, attachment of receptors on cell membrane proteins, and steroid metabolism. It is connected to the nuclear envelope. Smooth endoplasmic reticulum is found in a variety of cell types (both animal and plant), and it serves different functions in each. The Smooth ER also contains the enzyme glucose-6-phosphatase, which converts glucose-6-phosphateGlucose-6-phosphate
Glucose 6-phosphate is glucose sugar phosphorylated on carbon 6. This compound is very common in cells as the vast majority of glucose entering a cell will become phosphorylated in this way....
to glucose, a step in gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
. The SER consists of tubules and vesicles that branch forming a network. In some cells, there are dilated areas like the sacs of RER. The network of SER allows increased surface area for the action or storage of key enzymes and the products of these enzymes.
Sarcoplasmic reticulum
The sarcoplasmic reticulum (SR), from the Greek sarx, ("flesh"), is a special type of smooth ER found in smoothSmooth muscle
Smooth muscle is an involuntary non-striated muscle. It is divided into two sub-groups; the single-unit and multiunit smooth muscle. Within single-unit smooth muscle tissues, the autonomic nervous system innervates a single cell within a sheet or bundle and the action potential is propagated by...
and striated muscle
Striated muscle
Striated muscle tissue is a form of fibers that are combined into parallel fibers. More specifically, it can refer to:* Cardiac muscle .* Skeletal muscle* Branchiomeric muscles...
. The only structural difference between this organelle and the SER is the medley of proteins they have, both bound to their membranes and drifting within the confines of their lumens. This fundamental difference is indicative of their functions: The SER synthesizes molecules, while the SR stores and pumps calcium ions. The SR contains large stores of calcium, which it sequesters and then releases when the muscle cell is stimulated. The SR's release of calcium upon electrical stimulation of the cell plays a major role in excitation-contraction coupling
Excitation-contraction coupling
Excitation-contraction coupling is a term coined in 1952 to describe the physiological process of converting an electrical stimulus to a mechanical response . This process is fundamental to muscle physiology, whereby the electrical stimulus is usually an action potential and the mechanical...
.
Functions
The endoplasmic reticulum serves many general functions, including the facilitation of protein folding and the transport of synthesized proteins in sacs called cisternae.Correct folding of newly-made proteins is made possible by several endoplasmic reticulum chaperone proteins, including protein disulfide isomerase
Protein disulfide isomerase
Protein disulfide isomerase or PDI is an enzyme in the endoplasmic reticulum in eukaryotes that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold...
(PDI), ERp29, the Hsp70
Hsp70
The 70 kilodalton heat shock proteins are a family of ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms...
family member Grp78, calnexin
Calnexin
Calnexin is a 90kDa integral protein of the endoplasmic reticulum . It consists of a large N-terminal calcium-binding lumenal domain, a single transmembrane helix and a short , acidic cytoplasmic tail....
, calreticulin
Calreticulin
Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 is a protein that in humans is encoded by the CALR gene....
, and the peptidylpropyl isomerase family.
Only properly-folded proteins are transported from the rough ER to the Golgi complex.
Transport of proteins
Secretory proteins, mostly glycoproteins, are moved across the endoplasmic reticulum membrane. Proteins that are transported by the endoplasmic reticulum and from there throughout the cell are marked with an address tag called a signal sequenceSignal peptide
A signal peptide is a short peptide chain that directs the transport of a protein.Signal peptides may also be called targeting signals, signal sequences, transit peptides, or localization signals....
. The N-terminus (one end) of a polypeptide chain (i.e., a protein) contains a few amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s that work as an address tag, which are removed when the polypeptide reaches its destination. Proteins that are destined for places outside the endoplasmic reticulum are packed into transport vesicle
Vesicle (biology)
A vesicle is a bubble of liquid within another liquid, a supramolecular assembly made up of many different molecules. More technically, a vesicle is a small membrane-enclosed sack that can store or transport substances. Vesicles can form naturally because of the properties of lipid membranes , or...
s and moved along the cytoskeleton
Cytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
toward their destination.
The endoplasmic reticulum is also part of a protein sorting pathway. It is, in essence, the transportation system of the eukaryotic cell. The majority of endoplasmic reticulum resident proteins are retained in the endoplasmic reticulum through a retention motif. This motif is composed of four amino acids at the end of the protein sequence. The most common retention sequence is KDEL (lys-asp-glu-leu). However, variation on KDEL does occur and other sequences can also give rise to endoplasmic reticulum retention. It is not known whether such variation can lead to sub-endoplasmic reticulum localizations. There are three KDEL receptors in mammalian cells, and they have a very high degree of sequence identity. The functional difences between these receptors remain to be established.
Other functions
- Insertion of proteins into the endoplasmic reticulum membrane: Integral membrane proteinIntegral membrane proteinAn integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
s are inserted into the endoplasmic reticulum membrane as they are being synthesized (co-translational translocation). Insertion into the endoplasmic reticulum membrane requires the correct topogenic signal sequenceTopogenic sequenceA topogenic sequence is a segment of a protein that ensures it acquires the proper orientation during its insertion into the endoplasmic reticulum.A single internal topogenic sequence directs the insertion of some single-pass transmembrane proteins....
s in the protein. - Glycosylation: GlycosylationGlycosylationGlycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
involves the attachment of oligosaccharideOligosaccharideAn oligosaccharide is a saccharide polymer containing a small number of component sugars, also known as simple sugars...
s. - Disulfide bond formation and rearrangement: Disulfide bonds stabilize the tertiary and quaternary structure of many proteins.
- Drug metabolism: The smooth ER is the site at which some drugs are modified by microsomal enzymes, which include the cytochrome P450 enzymes.

