
Isoleucine
Encyclopedia
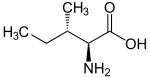
Isoleucine is an α-amino acid
with the chemical formula
HO2CCH(NH2)CH(CH3)CH2CH3. It is an essential amino acid
, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.
With a hydrocarbon side chain, isoleucine is classified as a hydrophobic amino acid. Together with threonine
, isoleucine is one of two common amino acids that have a chiral
side chain. Four stereoisomers of isoleucine are possible, including two possible diastereomer
s of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid.
and alpha-ketoglutarate. Enzymes involved in this biosynthesis include:
. In mammals Acetyl CoA cannot be converted back to carbohydrate but can be used in the synthesis of ketone bodies
or fatty acid
s, hence ketogenic.
Biotin
, sometimes referred to as Vitamin B7 or Vitamin H, is an absolute requirement for the full catabolism of isoleucine (as well as leucine
). Without adequate biotin, the human body will be unable to fully break down isoleucine and leucine molecules . This can lead to numerous physiological issues (related to muscle maintenance and protein synthesis, lipid metabolism, and fatty acid metabolism) as well as cognitive issues resulting from general metabolic pathway failure and the irritating effects of hydroxyisovalerate, a byproduct of incomplete isoleucine catabolism. Isovaleric acidemia
is an example of a disorder caused by incomplete catabolism of leucine.
and diethylmalonate. Synthetic isoleucine was originally reported in 1905.
German chemist Felix Ehrlich
discovered isoleucine in hemoglobin
in 1903.
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
HO2CCH(NH2)CH(CH3)CH2CH3. It is an essential amino acid
Essential amino acid
An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo by the organism , and therefore must be supplied in the diet.-Essentiality vs. conditional essentiality in humans:...
, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.
With a hydrocarbon side chain, isoleucine is classified as a hydrophobic amino acid. Together with threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, isoleucine is one of two common amino acids that have a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
side chain. Four stereoisomers of isoleucine are possible, including two possible diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid.
Biosynthesis
As an essential amino acid, isoleucine is not synthesized in animals, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvic acidPyruvic acid
Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways....
and alpha-ketoglutarate. Enzymes involved in this biosynthesis include:
- Acetolactate synthaseAcetolactate synthaseThe acetolactate synthase enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids ....
(also known as acetohydroxy acid synthase) - Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferaseValine-3-methyl-2-oxovalerate transaminaseIn enzymology, a valine-3-methyl-2-oxovalerate transaminase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are L-valine and -3-methyl-2-oxopentanoate, whereas its two products are 3-methyl-2-oxobutanoate and L-isoleucine.This enzyme belongs to the family...
Catabolism
Isoleucine is both a glucogenic and a ketogenic amino acid. After transamination with alpha-ketoglutarate the carbon skeleton can be converted into either Succinyl CoA, and fed into the TCA cycle for oxidation or conversion into oxaloacetate for gluconeogenesis (hence glucogenic). It can also be converted into Acetyl CoA and fed into the TCA cycle by condensing with oxaloacetate to form citrateCitrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
. In mammals Acetyl CoA cannot be converted back to carbohydrate but can be used in the synthesis of ketone bodies
Ketone bodies
Ketone bodies are three water-soluble compounds that are produced as by-products when fatty acids are broken down for energy in the liver and kidney. They are used as a source of energy in the heart and brain. In the brain, they are a vital source of energy during fasting...
or fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s, hence ketogenic.
Biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
, sometimes referred to as Vitamin B7 or Vitamin H, is an absolute requirement for the full catabolism of isoleucine (as well as leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
). Without adequate biotin, the human body will be unable to fully break down isoleucine and leucine molecules . This can lead to numerous physiological issues (related to muscle maintenance and protein synthesis, lipid metabolism, and fatty acid metabolism) as well as cognitive issues resulting from general metabolic pathway failure and the irritating effects of hydroxyisovalerate, a byproduct of incomplete isoleucine catabolism. Isovaleric acidemia
Isovaleric acidemia
Isovaleric acidemia, also called isovaleric aciduria or isovaleric acid CoA dehydrogenase deficiency, is a rare autosomal recessive metabolic disorder which disrupts or prevents normal metabolism of the branched-chain amino acid leucine...
is an example of a disorder caused by incomplete catabolism of leucine.
Nutritional Sources
Even though this amino acid is not produced in animals, it is stored in high quantities. Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.Isomers of isoleucine
| Forms of Isoleucine | |||||||
|---|---|---|---|---|---|---|---|
| Common name Common name A common name of a taxon or organism is a name in general use within a community; it is often contrasted with the scientific name for the same organism... : |
isoleucine | D-isoleucine | L-isoleucine | DL-isoleucine | allo-D-isoleucine | allo-L-isoleucine | allo-DL-isoleucine |
| Synonyms: | (R)-Isoleucine | L(+)-Isoleucine | (R*,R*)-isoleucine | alloisoleucine | |||
| PubChem PubChem PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information , a component of the National Library of Medicine, which is part of the United States National Institutes of Health . PubChem can... : |
|||||||
| EINECS number: | |||||||
| CAS number: | 443-79-8 | 319-78-8 | 73-32-5 | 1509-35-9 | 1509-34-8 | 3107-04-8 |
 |
| L-isoleucine (2S,3S) and D-isoleucine (2R,3R) |
| |
| L-allo-isoleucine (2S,3R) and D-allo-isoleucine (2R,3S) |
Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane2-Bromobutane
2-Bromobutane is an isomer of 1-bromobutane. Both compounds share the molecular formula C4H9Br. 2-Bromobutane is also known as sec-butyl bromide or methylethylbromomethane. Because it contains bromine, a halogen, it is part of a larger class of compounds known as alkyl halides. It is a...
and diethylmalonate. Synthetic isoleucine was originally reported in 1905.
German chemist Felix Ehrlich
Felix Ehrlich
Felix Ehrlich was a German chemist and biochemist.- Life and work :...
discovered isoleucine in hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
in 1903.

