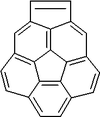
Corannulene
Encyclopedia
Corannulene is a polycyclic aromatic hydrocarbon
with chemical formula
C
20H
10. The molecule
consists of a cyclopentane
ring fused with 5 benzene
rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene
and can be considered a fragment of buckminsterfullerene
. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier
of 10.2 kcal
/mol
(42.7 kJ/mol) at −64 °C.
of an octabromide with potassium hydroxide
:
The bromine substituents are removed with an excess of n-butyllithium
.
Much effort is directed at functionalization of the corannulene ring with novel functional groups such as ethynyl groups, ether groups, thioether groups, platinum function groups, aryl groups, phenalenyl fused and indeno extensions.
for this compound is explained with a so-called annulene-within-an-annulene model. According to this model corannulene is made up of an aromatic 6 electron cyclopentadienyl anion
surrounded by an aromatic 14 electron annulenyl cation. This model was suggested by Barth and Lawton in the first synthesis of corannulene in 1966. They also suggested the trivial name 'corannulene', which is derived from the annulene-within-an-annulene model: core + annulene.
However, later theoretical calculations have disputed the validity of this approximation.
s. This has been performed with alkali metals, electrochemically and with bases. The corannulene dianion is antiaromatic and tetraanion is again aromatic. With lithium
as reducing agent
two tetraanions form a supramolecular dimer with two bowls stacked into each other with 4 lithium ions in between and 2 pairs above and below the stack.. This self-assembly motif was applied in the organization of fullerenes. Penta-substituted fullerenes (with methyl or phenyl groups) charged with five electrons form supramolecular dimers with a complementary corannulene tetraanion bowl, 'stitched' by interstitial lithium cations. In a related system 5 lithium ions are sandwiched between two corannulene bowls
In one cyclopenta[bc]corannulene a concave - concave aggregate is observed by NMR spectroscopy
with 2 C–Li–C bonds connecting the tetraanions.
Metals tend to bind to the convex face of the annulene. Concave binding has been reported for a cesium / crown ether system
Using electrospray ionization, a protonated corannulene cation has been produced in which the protonation site was observed to be on a peripheral sp2-carbon atom.
In this radical anion spin density is delocalized with a significant contribution from the central cyclopentadienyl radical.
s to form a corannulene carbocation
. Reaction with chloromethane
and aluminium chloride
results in the formation of an AlCl4- salt with a methyl group situated at the center with the cationic center at the rim. X-ray diffraction analysis shows the that the new carbon-carbon bond is elongated (157 pm)
When bicorannulenyl is reduced to a dianion with potassium metal, the central bond assumes significant double-bond character. This is due to its orbital structure, which has a LUMO orbital localized on the central bond.
When bicorannulenyl is reduced to an octaanion with lithium metal, it self-assembles into supramolecular oligomers.
This is based on the "charged polyarene stacking" self-assembly motif.
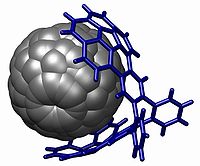 The corannulene group is used in host-guest chemistry
The corannulene group is used in host-guest chemistry
with interactions based on pi stacking , notably with fullerene
s (the buckycatcher) but also with nitrobenzene
With long aliphatic side chains corannulenes are reported forming a thermotropic hexagonal columnar liquid crystalline mesophase
. Corannulenes have also been used as the core group in a dendrimer
or as coordinating ligand
to metals. Corannulenes with ethynyl groups are investigated for their potential use as blue emitters.
Polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons , also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH...
with chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
20H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
10. The molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
consists of a cyclopentane
Cyclopentane
Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula 510 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C...
ring fused with 5 benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene
Geodesic polyarene
A geodesic polyarene in organic chemistry is a polycyclic aromatic hydrocarbon with curved convex or concave surfaces. Examples are fullerenes, nanotubes, corannulenes, helicenes and sumanene...
and can be considered a fragment of buckminsterfullerene
Buckminsterfullerene
Buckminsterfullerene is a spherical fullerene molecule with the formula . It was first intentionally prepared in 1985 by Harold Kroto, James Heath, Sean O'Brien, Robert Curl and Richard Smalley at Rice University...
. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier
Inversion barrier
In molecular geometry, the term inversion barrier refers to the amount of energy required for the geometric structure of a molecule to undergo the conformational change of inversion; i.e. for the molecule to be turned inside out. Nitrogen inversion is one example of such a transition in the...
of 10.2 kcal
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
(42.7 kJ/mol) at −64 °C.
Synthesis
Several synthetic routes exist to corannulene. Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives. Corannulane was first isolated in 1966 by multistep organic synthesis. A flash vacuum pyrolysis method followed in 1991. One synthesis based on solution chemistry consists of a nucleophilic displacement–elimination reactionElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of an octabromide with potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
:
The bromine substituents are removed with an excess of n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
.
Much effort is directed at functionalization of the corannulene ring with novel functional groups such as ethynyl groups, ether groups, thioether groups, platinum function groups, aryl groups, phenalenyl fused and indeno extensions.
Aromaticity
The observed aromaticityAromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
for this compound is explained with a so-called annulene-within-an-annulene model. According to this model corannulene is made up of an aromatic 6 electron cyclopentadienyl anion
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
surrounded by an aromatic 14 electron annulenyl cation. This model was suggested by Barth and Lawton in the first synthesis of corannulene in 1966. They also suggested the trivial name 'corannulene', which is derived from the annulene-within-an-annulene model: core + annulene.
However, later theoretical calculations have disputed the validity of this approximation.
Corannulene anions
Corannulene can be reduced up to a tetraanion in a series of one-electron reductionOne-electron reduction
A one-electron reduction in organic chemistry involves the transfer of an electron from a metal to an organic substrate. It serves to differentiate between true organic reductions and other reductions such as hydride transfer reactions that actually involve two-electron species.The first...
s. This has been performed with alkali metals, electrochemically and with bases. The corannulene dianion is antiaromatic and tetraanion is again aromatic. With lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
as reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
two tetraanions form a supramolecular dimer with two bowls stacked into each other with 4 lithium ions in between and 2 pairs above and below the stack.. This self-assembly motif was applied in the organization of fullerenes. Penta-substituted fullerenes (with methyl or phenyl groups) charged with five electrons form supramolecular dimers with a complementary corannulene tetraanion bowl, 'stitched' by interstitial lithium cations. In a related system 5 lithium ions are sandwiched between two corannulene bowls
In one cyclopenta[bc]corannulene a concave - concave aggregate is observed by NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
with 2 C–Li–C bonds connecting the tetraanions.
Metals tend to bind to the convex face of the annulene. Concave binding has been reported for a cesium / crown ether system
Corannulene cations
UV 193-nm photoionization effectively removes a π-electron from the twofold degenerate E1-HOMO located in the aromatic network of electrons yielding a corannulene radical cation. Owing to the degeneracy in the HOMO orbital, the corannulene radical cation is unstable in its original C5v molecular arrangement, and therefore, subject to Jahn-Teller (JT) vibronic distortion.Using electrospray ionization, a protonated corannulene cation has been produced in which the protonation site was observed to be on a peripheral sp2-carbon atom.
Corannulene radicals
Corannulene-based free radicals have also been synthesised and studied. A semiquinone radical anion obtained by reduction of the diketone by a sodium mirror (see below) has been reported stable in the solid state- .
In this radical anion spin density is delocalized with a significant contribution from the central cyclopentadienyl radical.
Corannulene carbocations
Corannulene can react with electrophileElectrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s to form a corannulene carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
. Reaction with chloromethane
Chloromethane
Chloromethane, also called methyl chloride, R-40 or HCC 40, is a chemical compound of the group of organic compounds called haloalkanes. It was once widely used as a refrigerant. It is a colorless extremely flammable gas with a minorly sweet odor, which is, however, detected at possibly toxic levels...
and aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
results in the formation of an AlCl4- salt with a methyl group situated at the center with the cationic center at the rim. X-ray diffraction analysis shows the that the new carbon-carbon bond is elongated (157 pm)
Bicorannulenyl
Bicorannulenyl is the corannulene dimer, in which two corannulene units are connected through a single bond. The molecule's stereochemistry consists of two chiral elements: the asymmetry of a singly substituted corannulenyl, and the helical twist about the central bond. In the neutral state, bicorannulenyl exists as 12 conformers, which intercovert through multiple bowl-inversions and bond-rotations.When bicorannulenyl is reduced to a dianion with potassium metal, the central bond assumes significant double-bond character. This is due to its orbital structure, which has a LUMO orbital localized on the central bond.
When bicorannulenyl is reduced to an octaanion with lithium metal, it self-assembles into supramolecular oligomers.
This is based on the "charged polyarene stacking" self-assembly motif.
Applications

Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
with interactions based on pi stacking , notably with fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s (the buckycatcher) but also with nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
With long aliphatic side chains corannulenes are reported forming a thermotropic hexagonal columnar liquid crystalline mesophase
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
. Corannulenes have also been used as the core group in a dendrimer
Dendrimer
Dendrimers are repetitively branched molecules. The name comes from the Greek word "δένδρον" , which translates to "tree". Synonymous terms for dendrimer include arborols and cascade molecules. However, dendrimer is currently the internationally accepted term. A dendrimer is typically symmetric...
or as coordinating ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
to metals. Corannulenes with ethynyl groups are investigated for their potential use as blue emitters.