
Solubility equilibrium
Encyclopedia
Solubility equilibrium is a type of dynamic equilibrium
. It exists when a chemical compound
in the solid state is in chemical equilibrium
with a solution
of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solvent, such as acid or alkali. Each type of equilibrium is characterized by a temperature-dependent equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
in the solid state is in chemical equilibrium
with a solution
of that compound. The equilibrium is an example of dynamic equilibrium
in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution
and precipitation
are equal to one another. When equilibrium is established, the solution is said to be saturated. The concentration
of the solute in a saturated solution is known as the solubility
. Units of solubility may be molar (mol dm−3) or expressed as mass per unit volume, such as μg ml−1. Solubility is temperature dependent. A solution containing a higher concentration of solute than the solubility is said to be supersaturated. A supersaturated solution may be induced to come to equilibrium by the addition of a "seed" which may be a tiny crystal of the solute, or a tiny solid particle, which initiates precipitation.
There are three main types of solubility equilibria.
In each case an equilibrium constant can be specified as a quotient of activities
. This equilibrium constant is dimensionless as activity is a dimensionless quantity. However, use of activities is very inconvenient, so the equilibrium constant is usually divided by the quotient of activity coefficients, to become a quotient of concentrations. See equilibrium chemistry#Equilibrium constant for details. Moreover, the concentration of solvent is usually taken to be constant and so is also subsumed into the equilibrium constant. For these reasons, the constant for a solubility equilibrium has dimensions related to the scale on which concentrations are measured. Solubility constants defined in terms of concentrations are not only temperature dependent, but also may depend on solvent composition when the solvent contains also species other than those derived from the solute.
. Therefore, the solubility product is expected to be different depending on the phase of the solid. For example, aragonite
and calcite
will have different solubility products even though they have both the same chemical identity (calcium carbonate
). Nevertheless, under given conditions, most likely only one phase is thermodynamically stable and therefore this phase enters a true equilibrium.
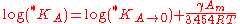
where is the solubility constant for the solute particles with the molar surface area A,
is the solubility constant for the solute particles with the molar surface area A,  is the solubility constant for substance with molar surface area tending to zero (i.e., when the particles are large), γ is the surface tension
is the solubility constant for substance with molar surface area tending to zero (i.e., when the particles are large), γ is the surface tension
of the solute particle in the solvent, Am is the molar surface area of the solute (in m2/mol), R is the universal gas constant, and T is the absolute temperature.
of the solution and hence on activity coefficient
s, so that the equilibrium constant, expressed as a concentration quotient, changes.
 Solubility is sensitive to changes in temperature
Solubility is sensitive to changes in temperature
. For example, sugar is more soluble in hot water than cool water. It occurs because solubility constants, like other types of equilibrium constants, are functions of temperature. In accordance with Le Chatelier's Principle
, when the dissolution process is endothermic (heat is absorbed), solubility increases with rising temperature, but when the process is exothermic
(heat is released) solubility decreases with rising temperature. The temperature effect is the basis for the process of recrystallization
, which can be used to purify a chemical compound.
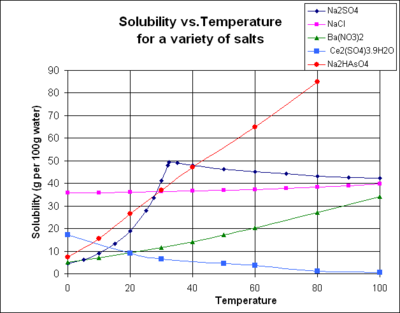
Sodium sulfate
shows increasing solubility with temperature below about 32.4°C, but a decreasing solubility at higher temperature. This is because the solid phase is the decahydrate, Na2SO4.10H2O, below the transition temperature but a different hydrate above that temperature.

where the index i iterates the components, Ni is the mole fraction of the ith component in the solution, P is the pressure, the index T refers to constant temperature, Vi,aq is the partial molar volume of the ith component in the solution, Vi,cr is the partial molar volume of the ith component in the dissolving solid, and R is the universal gas constant.
The pressure dependence of solubility does occasionally have practical significance. For example, precipitation fouling of oil fields and wells by calcium sulfate (which decreases its solubility with decreasing pressure) can result in decreased productivity with time.
(table sugar) forms a saturated solution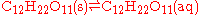 .
.
An equilibrium expression for this reaction can be written, as for any chemical reaction (products over reactants):
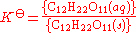
where K is called the thermodynamic solubility constant. The braces indicate activity
is called the thermodynamic solubility constant. The braces indicate activity
. The activity of a pure solid is, by definition, unity. Therefore
The activity of a substance, A, in solution can be expressed as the product of the concentration, [A], and an activity coefficient
, γ. When K is divided by γ the solubility constant, Ks,
is divided by γ the solubility constant, Ks,
is obtained. This is equivalent to defining the standard state
as the saturated solution so that the activity coefficient is equal to one. The solubility constant is a true constant only if the activity coefficient is not affected by the presence of any other solutes that may be present. The unit of the solubility constant is the same as the unit of the concentration of the solute. For sucrose
K = 1.971 mol dm−3 at 25 °C. This shows that the solubility of sucrose at 25 °C is nearly 2 mol dm−3 (540 g/l). Sucrose is an unusual in that it does not easily form a supersaturated solution at higher concentrations, as do most other carbohydrate
s.
into their constituent ions when they dissolve in water. For example, for calcium sulfate
:

As for the previous example, the equilibrium expression is:

where K is the thermodynamic equilibrium constant and braces indicate activity. The activity of a pure solid is, by definition, equal to one.
is the thermodynamic equilibrium constant and braces indicate activity. The activity of a pure solid is, by definition, equal to one.
When the solubility of the salt is very low the activity coefficients of the ions in solution are nearly equal to one. By setting them to be actually equal to one this expression reduces to the solubility product expression:
The solubility product for a general binary compound ApBq is given by
When the product dissociates the concentration of B is equal to q/p times the concentration of A.
Therefore
The solubility, S is 1/p [A]. One may incorporate 1/p and insert it under the root to obtain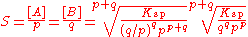
Examples
Solubility products are often expressed in logarithmic form. Thus, for calcium sulfate, Ksp = , log Ksp = -4.32. The smaller the value, or the more negative the log value, the lower the solubility.
Some salts are not fully dissociated in solution. Examples include MgSO4
, famously discovered by Manfred Eigen
to be present in seawater
as both an inner sphere complex
and an outer sphere complex. The solubility of such salts is calculated by the method outlined in dissolution with reaction.
constant for water, Kw.
For example,
log Ksp for Ca(OH)2 is about -5 at ambient temperatures; log K*sp = -5 + 2 × 14 = 23, approximately.
is the effect of decreasing the solubility of one salt, when another salt, which has an ion in common with it, is also present. For example, the solubility of silver chloride
, AgCl, is lowered when sodium chloride, a source of the common ion chloride, is added to a suspension of AgCl in water.
The solubility, S, in the absence of a common ion can be calculated as follows. The concentrations [Ag+] and [Cl-] are equal because one mole of AgCl dissociates into one mole of Ag+ and one mole of Cl-. Let the concentration of [Ag+](aq) be denoted by x.
Ksp for AgCl is equal to mol2dm−6 at 25°C, so the solubility is mol dm−3.
Now suppose that sodium chloride is also present , at a concentration of 0.01 mol dm−3. The solubility, ignoring any possible effect of the sodium ions, is now calculated by
This is a quadratic equation in x, which is also equal to the solubility.
In the case of silver chloride x2 is very much smaller than 0.01 x, so this term can be ignored. Therefore
a considerable reduction. In gravimetric analysis
for silver, the reduction in solubility due to the common ion effect is used to ensure "complete" precipitation of AgCl.
, B, dissolving in an acidic aqueous solution.
This reaction is very important for pharmaceutical products. Dissolution of weak acids in alkaline media is similarly important.
The uncharged molecule usually has lower solubility than the ionic form, so solubility depends on pH and the acid dissociation constant
of the solute. The term "intrinsic solubility" is used to describe the solubility of the un-ionized form in the absence of acid or alkali.
Leaching of aluminium salts from rocks and soil by acid rain
is another example of dissolution with reaction: alumino-silicates are bases which react with the acid to form soluble species, such as Al3+(aq).
Formation of a chemical complex
may also change solubility. A well-known example, is the addition of a concentrated solution of ammonia
to a suspension of silver chloride
, in which dissolution is favoured by the formation of an ammine complex.
Another example involves the addition of water softeners to washing powders to inhibit the precipitation of salts of magnesium and calcium ions, which are present in hard water
, by forming complexes with them.
The calculation of solubility in these cases requires two or more simultaneous equilibria to be considered. For example,
A number of computer programs are available to do the calculations. They include:
A variation of the static method is to add a solution of the substance in a non-aqueous solvent, such as dimethyl sulfoxide
, to an aqueous buffer
mixture. Immediate precipitation may occur giving a cloudy mixture. The solubility measured for such a mixture is known as "kinetic solubility". The cloudiness is due to the fact that the precipitate particles are very small resulting in Tyndall scattering. In fact the particles are so small that the particle size effect comes into play and kinetic solubility is often greater than equilibrium solubility. Over time the cloudiness will disappear as the size of the crystallites increases, and eventually equilibrium will be reached in a process known as precipitate ageing.
Dynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
. It exists when a chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
in the solid state is in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solvent, such as acid or alkali. Each type of equilibrium is characterized by a temperature-dependent equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
Definitions
A solubility equilibrium exists when a chemical compoundChemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
in the solid state is in chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
of that compound. The equilibrium is an example of dynamic equilibrium
Dynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution
Dissolution (chemistry)
Dissolution is the process by which a solid, liquid or gas forms a solution in a solvent. In solids this can be explained as the breakdown of the crystal lattice into individual ions, atoms or molecules and their transport into the solvent. For liquids and gases, the molecules must be compatible...
and precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
are equal to one another. When equilibrium is established, the solution is said to be saturated. The concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of the solute in a saturated solution is known as the solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
. Units of solubility may be molar (mol dm−3) or expressed as mass per unit volume, such as μg ml−1. Solubility is temperature dependent. A solution containing a higher concentration of solute than the solubility is said to be supersaturated. A supersaturated solution may be induced to come to equilibrium by the addition of a "seed" which may be a tiny crystal of the solute, or a tiny solid particle, which initiates precipitation.
There are three main types of solubility equilibria.
- Simple dissolution.
- Dissolution with dissociation. This is characteristic of salts. The equilibrium constant is known in this case as a solubility product.
- Dissolution with reaction. This is characteristic of the dissolution of weak acidWeak acidA weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
s or weak baseWeak baseIn chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted–Lowry bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH compared to strong bases...
s in aqueous media of varying pHPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
.
In each case an equilibrium constant can be specified as a quotient of activities
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
. This equilibrium constant is dimensionless as activity is a dimensionless quantity. However, use of activities is very inconvenient, so the equilibrium constant is usually divided by the quotient of activity coefficients, to become a quotient of concentrations. See equilibrium chemistry#Equilibrium constant for details. Moreover, the concentration of solvent is usually taken to be constant and so is also subsumed into the equilibrium constant. For these reasons, the constant for a solubility equilibrium has dimensions related to the scale on which concentrations are measured. Solubility constants defined in terms of concentrations are not only temperature dependent, but also may depend on solvent composition when the solvent contains also species other than those derived from the solute.
Phase effect
Equilibria are defined for specific crystal phasesPhase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
. Therefore, the solubility product is expected to be different depending on the phase of the solid. For example, aragonite
Aragonite
Aragonite is a carbonate mineral, one of the two common, naturally occurring, crystal forms of calcium carbonate, CaCO3...
and calcite
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate . The other polymorphs are the minerals aragonite and vaterite. Aragonite will change to calcite at 380-470°C, and vaterite is even less stable.-Properties:...
will have different solubility products even though they have both the same chemical identity (calcium carbonate
Calcium carbonate
Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime,...
). Nevertheless, under given conditions, most likely only one phase is thermodynamically stable and therefore this phase enters a true equilibrium.
Particle size effect
The thermodynamic solubility constant is defined for large monocrystals. Solubility will increase with decreasing size of solute particle (or droplet) because of the additional surface energy. This effect is generally small unless particles become very small, typically smaller than 1 μm. The effect of the particle size on solubility constant can be quantified as follows: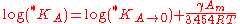
where
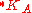 is the solubility constant for the solute particles with the molar surface area A,
is the solubility constant for the solute particles with the molar surface area A, 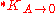 is the solubility constant for substance with molar surface area tending to zero (i.e., when the particles are large), γ is the surface tension
is the solubility constant for substance with molar surface area tending to zero (i.e., when the particles are large), γ is the surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
of the solute particle in the solvent, Am is the molar surface area of the solute (in m2/mol), R is the universal gas constant, and T is the absolute temperature.
Salt effect
The salt effect refers to the fact that the presence of a salt which has no ion in common with the solute, has an effect on the ionic strengthIonic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
of the solution and hence on activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
s, so that the equilibrium constant, expressed as a concentration quotient, changes.
Temperature effect

Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. For example, sugar is more soluble in hot water than cool water. It occurs because solubility constants, like other types of equilibrium constants, are functions of temperature. In accordance with Le Chatelier's Principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
, when the dissolution process is endothermic (heat is absorbed), solubility increases with rising temperature, but when the process is exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
(heat is released) solubility decreases with rising temperature. The temperature effect is the basis for the process of recrystallization
Recrystallization (chemistry)
-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
, which can be used to purify a chemical compound.
Sodium sulfate
Sodium sulfate
Sodium sulfate is the sodium salt of sulfuric acid. When anhydrous, it is a white crystalline solid of formula Na2SO4 known as the mineral thenardite; the decahydrate Na2SO4·10H2O has been known as Glauber's salt or, historically, sal mirabilis since the 17th century. Another solid is the...
shows increasing solubility with temperature below about 32.4°C, but a decreasing solubility at higher temperature. This is because the solid phase is the decahydrate, Na2SO4.10H2O, below the transition temperature but a different hydrate above that temperature.
Pressure effect
For condensed phases (solids and liquids), the pressure dependence of solubility is typically weak and usually neglected in practice. Assuming an ideal solution, the dependence can be quantified as:
where the index i iterates the components, Ni is the mole fraction of the ith component in the solution, P is the pressure, the index T refers to constant temperature, Vi,aq is the partial molar volume of the ith component in the solution, Vi,cr is the partial molar volume of the ith component in the dissolving solid, and R is the universal gas constant.
The pressure dependence of solubility does occasionally have practical significance. For example, precipitation fouling of oil fields and wells by calcium sulfate (which decreases its solubility with decreasing pressure) can result in decreased productivity with time.
Simple dissolution
Dissolution of an organic solid can be described as an equilibrium between the substance in its solid and dissolved forms. For example, when sucroseSucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
(table sugar) forms a saturated solution
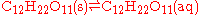 .
.An equilibrium expression for this reaction can be written, as for any chemical reaction (products over reactants):
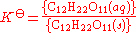
where K
 is called the thermodynamic solubility constant. The braces indicate activity
is called the thermodynamic solubility constant. The braces indicate activityActivity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
. The activity of a pure solid is, by definition, unity. Therefore

The activity of a substance, A, in solution can be expressed as the product of the concentration, [A], and an activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
, γ. When K
 is divided by γ the solubility constant, Ks,
is divided by γ the solubility constant, Ks,
is obtained. This is equivalent to defining the standard state
Standard state
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry recommends a conventional set of standard states...
as the saturated solution so that the activity coefficient is equal to one. The solubility constant is a true constant only if the activity coefficient is not affected by the presence of any other solutes that may be present. The unit of the solubility constant is the same as the unit of the concentration of the solute. For sucrose
Sucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
K = 1.971 mol dm−3 at 25 °C. This shows that the solubility of sucrose at 25 °C is nearly 2 mol dm−3 (540 g/l). Sucrose is an unusual in that it does not easily form a supersaturated solution at higher concentrations, as do most other carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s.
Dissolution with dissociation
Ionic compounds normally dissociateDissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
into their constituent ions when they dissolve in water. For example, for calcium sulfate
Calcium sulfate
Calcium sulfate is a common laboratory and industrial chemical. In the form of γ-anhydrite , it is used as a desiccant. It is also used as a coagulant in products like tofu. In the natural state, unrefined calcium sulfate is a translucent, crystalline white rock...
:

As for the previous example, the equilibrium expression is:

where K
 is the thermodynamic equilibrium constant and braces indicate activity. The activity of a pure solid is, by definition, equal to one.
is the thermodynamic equilibrium constant and braces indicate activity. The activity of a pure solid is, by definition, equal to one.When the solubility of the salt is very low the activity coefficients of the ions in solution are nearly equal to one. By setting them to be actually equal to one this expression reduces to the solubility product expression:

The solubility product for a general binary compound ApBq is given by
- ApBq pAq+ + qBp-
- Ksp = [A]p[B]q (electrical charges omitted for simplicity of notation)
When the product dissociates the concentration of B is equal to q/p times the concentration of A.
- [B] = q/p [A]
Therefore
- Ksp = [A]p (q/p)q [A]q
- =(q/p)q × [A]p+q
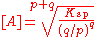
The solubility, S is 1/p [A]. One may incorporate 1/p and insert it under the root to obtain
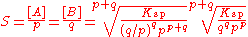
Examples
- CaSO4: p=1, q=1,

- Na2SO4: p=2, q=1,
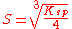
- Al2(SO4)3: p=2, q=3,
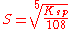
Solubility products are often expressed in logarithmic form. Thus, for calcium sulfate, Ksp = , log Ksp = -4.32. The smaller the value, or the more negative the log value, the lower the solubility.
Some salts are not fully dissociated in solution. Examples include MgSO4
Magnesium sulfate
Magnesium sulfate is a chemical compound containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate epsomite , commonly called Epsom salt, from the town of Epsom in Surrey, England, where the salt was distilled from the springs that arise where the...
, famously discovered by Manfred Eigen
Manfred Eigen
Manfred Eigen is a German biophysical chemist who won the 1967 Nobel Prize in Chemistry for work on measuring fast chemical reactions.-Career:...
to be present in seawater
Seawater
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% . This means that every kilogram of seawater has approximately of dissolved salts . The average density of seawater at the ocean surface is 1.025 g/ml...
as both an inner sphere complex
Inner sphere complex
Inner sphere complex is a type of surface complex. Formation of inner sphere complexes occurs when ions bind directly to the surface with no intervening water molecules. These types of surface complexes are restricted to ions that have a high affinity for surface sites and include specifically...
and an outer sphere complex. The solubility of such salts is calculated by the method outlined in dissolution with reaction.
Hydroxides
For hydroxides solubility products are often given in a modified form, K*sp, using hydrogen ion concentration in place of hydroxide ion concentration. The two concentrations are related by the self-ionizationSelf-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
constant for water, Kw.
- Kw=[H+][OH-]
For example,
- Ca(OH)2 Ca2+ + 2 OH-
- Ksp = [Ca2+][OH-]2 = [Ca2+]Kw2[H+]-2
- K*sp = Ksp/Kw2 = [Ca2+][H+]-2
log Ksp for Ca(OH)2 is about -5 at ambient temperatures; log K*sp = -5 + 2 × 14 = 23, approximately.
Common ion effect
The common-ion effectCommon-ion effect
The common ion effect is an effect which results when two substances, which both ionize to give the same ion, are involved in a chemical equilibrium.-Solubility effects:...
is the effect of decreasing the solubility of one salt, when another salt, which has an ion in common with it, is also present. For example, the solubility of silver chloride
Silver chloride
Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water . Upon illumination or heating, silver chloride converts to silver , which is signalled by greyish or purplish coloration to some samples...
, AgCl, is lowered when sodium chloride, a source of the common ion chloride, is added to a suspension of AgCl in water.
- AgCl(s) Ag+(aq) + Cl-(aq); Ksp = [Ag+][Cl-]
The solubility, S, in the absence of a common ion can be calculated as follows. The concentrations [Ag+] and [Cl-] are equal because one mole of AgCl dissociates into one mole of Ag+ and one mole of Cl-. Let the concentration of [Ag+](aq) be denoted by x.
- Ksp = x2; S = x =

Ksp for AgCl is equal to mol2dm−6 at 25°C, so the solubility is mol dm−3.
Now suppose that sodium chloride is also present , at a concentration of 0.01 mol dm−3. The solubility, ignoring any possible effect of the sodium ions, is now calculated by
- Ksp = x(0.01 + x)
This is a quadratic equation in x, which is also equal to the solubility.
- x2 + 0.01 x - Ksp = 0
In the case of silver chloride x2 is very much smaller than 0.01 x, so this term can be ignored. Therefore
- S = x = Ksp / 0.01 = mol dm-3,
a considerable reduction. In gravimetric analysis
Gravimetric analysis
Gravimetric analysis describes a set of methods in analytical chemistry for the quantitative determination of an analyte based on the mass of a solid...
for silver, the reduction in solubility due to the common ion effect is used to ensure "complete" precipitation of AgCl.
Dissolution with reaction
A typical reaction with dissolution involves a weak baseWeak base
In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted–Lowry bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH compared to strong bases...
, B, dissolving in an acidic aqueous solution.
- B(s) + H+ (aq) BH+ (aq)
This reaction is very important for pharmaceutical products. Dissolution of weak acids in alkaline media is similarly important.
- HnA(s) + OH-(aq) Hn-1A-(aq) + H2O
The uncharged molecule usually has lower solubility than the ionic form, so solubility depends on pH and the acid dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of the solute. The term "intrinsic solubility" is used to describe the solubility of the un-ionized form in the absence of acid or alkali.
Leaching of aluminium salts from rocks and soil by acid rain
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions . It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of carbon dioxide, sulfur dioxide and nitrogen...
is another example of dissolution with reaction: alumino-silicates are bases which react with the acid to form soluble species, such as Al3+(aq).
Formation of a chemical complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
may also change solubility. A well-known example, is the addition of a concentrated solution of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
to a suspension of silver chloride
Silver chloride
Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water . Upon illumination or heating, silver chloride converts to silver , which is signalled by greyish or purplish coloration to some samples...
, in which dissolution is favoured by the formation of an ammine complex.
- AgCl(s) +2 NH3(aq) [Ag(NH3)2]+ (aq) + Cl- (aq)
Another example involves the addition of water softeners to washing powders to inhibit the precipitation of salts of magnesium and calcium ions, which are present in hard water
Hard water
Hard water is water that has high mineral content . Hard water has high concentrations of Ca2+ and Mg2+ ions. Hard water is generally not harmful to one's health but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling...
, by forming complexes with them.
The calculation of solubility in these cases requires two or more simultaneous equilibria to be considered. For example,
| Intrinsic solubility equilibrium | B(s) B(aq): Ks = [B(aq)] |
| Acid-base equilibrium | B(aq) + H+(aq) BH+(aq) Ka = [B(aq)][H+(aq)]/[BH+(aq)] |
A number of computer programs are available to do the calculations. They include:
- Geochem-EZ (freeware) a multi-purpose chemical speciation program, used in plant nutrition and in soil and environmental chemistry research to perform equilibrium speciation computations, allowing the user to estimate solution ion activities and to consider simple complexes and solid phases.
- HySS (freeware) which was used to produce the diagram at the right.
- CHEMEQL A comprehensive computer program for the calculation of thermodynamic equilibrium concentrations of species in homogeneous and heterogeneous systems. Many geochemical applications.
- WinSGW A Windows version of the SOLGASWATER computer program.
- Visual MINTEQ A Windows version of MINTEQA2 (ver 4.0). MINTEQA2 is a chemical equilibrium model for the calculation of metal speciation, solubility equilibria etc. for natural waters.
- MINEQL+ A chemical equilibrium modeling system for aqueous systems. Handles a wide range of pH, redox, solubility and sorption scenarios.
- JESS All types of chemical equilibria can be modelled including protonation, complex formation, redox, solubility and adsorption interactions. Includes an extensive database.
Experimental determination
The determination of solubility is fraught with difficulties. First and foremost is the difficulty in establishing that the system is in equilibrium at the chosen temperature. This is because both precipitation and dissolution reactions may be extremely slow. If the process is very slow solvent evaporation may be an issue. Supersaturation may occur. With very insoluble substances, the concentrations in solution are very low and difficult to determine. The methods used fall broadly into two categories, static and dynamic.Static methods
In static methods a mixture is brought to equilibrium and the concentration of a species in the solution phase is determined by chemical analysis. This usually requires separation of the solid and solution phases. In order to do this the equilibration and separation should be performed in a thermostatted room. Very low concentrations can be measured if a radioactive tracer is incorporated in the solid phase.A variation of the static method is to add a solution of the substance in a non-aqueous solvent, such as dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, to an aqueous buffer
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
mixture. Immediate precipitation may occur giving a cloudy mixture. The solubility measured for such a mixture is known as "kinetic solubility". The cloudiness is due to the fact that the precipitate particles are very small resulting in Tyndall scattering. In fact the particles are so small that the particle size effect comes into play and kinetic solubility is often greater than equilibrium solubility. Over time the cloudiness will disappear as the size of the crystallites increases, and eventually equilibrium will be reached in a process known as precipitate ageing.
Dynamic methods
Solubility values of organic acids, bases, and ampholytes of pharmaceutical interest may be obtained by a proceess called "Chasing equilibrium solubility". In this procedure, a quantity of substance is first dissolved at a pH where it exists predominantly in its ionized form and then a precipitate of the neutral (un-ionized) species is formed by changing the pH. Subsequently, the rate of change of pH due to precipitation or dissolution is monitored and strong acid and base titrant are added to adjust the pH to discover the equilibrium conditions when the two rates are equal. The advantage of this method is that it is relatively fast as the quantity of precipitate formed is quite small. However, the performance of the method may be affected by the formation supersaturated solutions.See also
- Solubility tableSolubility tableThe table below provides information on the variation of solubility of different substances in water with temperature, under 1 atm pressure, units of solubility in g/100g H2O...
A table of solubilities of mostly inorganic salts at temperatures between 0 and 100oC Section 6.9 Solubilities of ionic salts. Includes a discussion of the thermodynamics of dissolution.
External links
- IUPAC-NIST solubility database
- Solubility products of simple inorganic compounds
- Solubility challenge Predict solubilities from a data base of 100 molecules. The database, of mostly compounds of pharmaceutical interest, is available at One hundred molecules with solubilities (Text file, tab separated).

