Semimetal
Encyclopedia
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
. For insulators, the magnitude of the band gap is larger (e.g. > 4 eV) than that of a semiconductor (e.g. < 4 eV). Metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s have a partially filled conduction band. A semimetal is a material with a very small overlap between the bottom of the conduction band
Electronic band structure
In solid-state physics, the electronic band structure of a solid describes those ranges of energy an electron is "forbidden" or "allowed" to have. Band structure derives from the diffraction of the quantum mechanical electron waves in a periodic crystal lattice with a specific crystal system and...
and the top of the valence band
Valence band
In solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
. A semimetal thus has no band gap and a negligible density of states
Density of states
In solid-state and condensed matter physics, the density of states of a system describes the number of states per interval of energy at each energy level that are available to be occupied by electrons. Unlike isolated systems, like atoms or molecules in gas phase, the density distributions are not...
at the Fermi level
Fermi level
The Fermi level is a hypothetical level of potential energy for an electron inside a crystalline solid. Occupying such a level would give an electron a potential energy \epsilon equal to its chemical potential \mu as they both appear in the Fermi-Dirac distribution function,which...
. A metal, by contrast, has an appreciable density of states at the Fermi level because the conduction band is partially filled.
The insulating/semiconducting states differ from the semimetallic/metallic states in the temperature dependency of their electrical conductivity. With a metal (which has only one type of charge carrier - electrons), the conductivity decreases with increases in temperature (due to increasing interaction of electrons with phonons (lattice vibrations)). With an insulator or semiconductor (which have two types of charge carriers - holes and electrons), both the carrier mobilities and carrier concentrations will contribute to the conductivity and these have different temperature dependencies. Ultimately, it is observed that the conductivity of insulators and semiconductors increase with initial increases in temperature above absolute zero (as more electrons are shifted to the conduction band), before decreasing with intermediate temperatures and then, once again, increasing with still higher temperatures. The semimetallic state is similar to the metallic state but in semimetals both holes and electrons contribute to electrical conduction. With some semimetals, like arsenic and antimony, there is a temperature-independent carrier density below room temperature (as in metals) while, in bismuth, this is true at very low temperatures but at higher temperatures the carrier density increases with temperature giving rise to a semimetal-semiconductor transition. A semimetal also differs from an insulator or semiconductor in that a semimetal's conductivity is always non-zero, whereas a semiconductor has zero conductivity at zero temperature and insulators have zero conductivity even at ambient temperatures (due to a wider band gap).
To classify semiconductors and semimetals, the energies of their filled and empty bands must be plotted against the crystal momentum of conduction electrons. According to the Bloch theorem the conduction of electrons depends on the periodicity of the crystal lattice in different directions.
In a semimetal, the bottom of the conduction band is typically situated in a different part of momentum space (at a different k-vector
Wave vector
In physics, a wave vector is a vector which helps describe a wave. Like any vector, it has a magnitude and direction, both of which are important: Its magnitude is either the wavenumber or angular wavenumber of the wave , and its direction is ordinarily the direction of wave propagation In...
) than the top of the valence band. One could say that a semimetal is a semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
with a negative indirect bandgap, although they are seldom described in those terms.
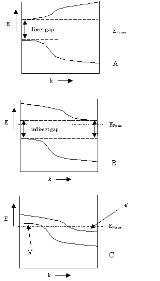
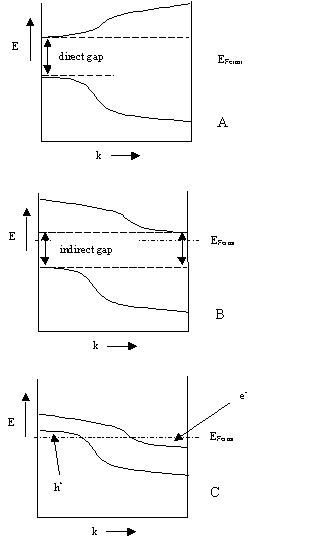
Schematically, the figure shows
- A) a semiconductor with a direct gap (like e.g. CuInSe2),
- B) a semiconductor with an indirect gap (like Si) and
- C) a semimetal (like Sn or graphite and the divalent Group IIA elements).
The figure is schematic, showing only the lowest-energy conduction band and the highest-energy valence band in one dimension of momentum space (or k-space). In typical solids, k-space is three dimensional, and there are an infinite number of bands.
Unlike a regular metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
, semimetals have charge carriers of both types (holes and electrons), so that one could also argue that they should be called 'double-metals' rather than semimetals. However, the charge carriers typically occur in much smaller numbers than in a real metal. In this respect they resemble degenerate semiconductor
Degenerate semiconductor
A degenerate semiconductor is a semiconductor with such a high level of doping that the material starts to act more like a metal than as a semiconductor....
s more closely. This explains why the electrical properties of semimetals are partway between those of metals and semiconductors.
As semimetals have fewer charge carriers than metals, they typically have lower electrical and thermal conductivities
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
. They also have small effective masses for both holes and electrons because the overlap in energy is usually the result of the fact that both energy bands are broad. In addition they typically show high diamagnetic susceptibilities and high lattice dielectric constants.
The classic semimetallic elements are arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
, antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, α-tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
(gray tin) and graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, an allotrope of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
. The first two (As, Sb) are also considered metalloids but the terms semimetal and metalloid are not synonymous. Semimetals, in contrast to metalloids, can also be compounds, such as HgTe, and tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, and graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
are typically not considered metalloids.
Transient semimetal states have been reported at extreme conditions.
See also
- Solid-state physicsSolid-state physicsSolid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from...
- Charge transfer insulatorsCharge transfer insulatorsCharge transfer insulators are a class of materials predicted to be conductors following conventional band theory, but which are in fact insulators due to a charge transfer process. Unlike Mott insulators, where the insulating properties arise from electrons hopping between unit cells, the...
- Mott insulators
- Hubbard modelHubbard modelThe Hubbard model is an approximate model used, especially in solid state physics, to describe the transition between conducting and insulating systems...
- Half-metal

