
Robinson annulation
Encyclopedia
The Robinson annulation is an organic reaction
used to create a six-member ring α,β-unsaturated cyclic ketone, using a ketone
(or aldehyde
) and methyl vinyl ketone
. It is named after Sir Robert Robinson, the British chemist who discovered it while he was at the University of Oxford
.
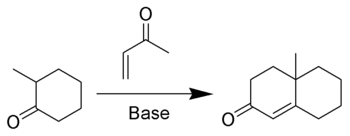 In addition to methyl vinyl ketone, 1-chloro-3-butanone and isoxazole
In addition to methyl vinyl ketone, 1-chloro-3-butanone and isoxazole
s will give the same product.
The Wieland-Miescher ketone
is the Robinson annulation product of 2-methyl-1,3-cyclohexanedione and methyl vinyl ketone
while the Hajos-Parrish ketone is the product of 2-methyl-1,3-cyclopentanedione and methyl vinyl ketone
. Asymmetric synthesis of these compounds has greatly increased their synthetic utility.
as they are simultaneously a Michael acceptor and able to take part in an aldol condensation
. The first step in the Robinson annulation (also spelt annelation) is a Michael addition
followed by an aldol reaction
as the annulation
step in the process. The reaction then proceeds as an aldol condensation to make the desired cyclohexenone
ring.

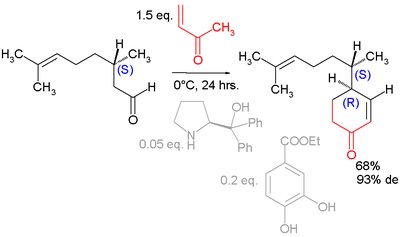
has been used to resolve the enantiomer
ic isomer
s of Robinson annulation
s in asymmetric synthesis. A proline derivative was employed in an asymmetric annulation of a geranial :
. The Hauser donor is an aromatic methylene sulfoxide
or sulfone
with a carboxylic ester group in the ortho position
. The Hauser acceptor is a Michael acceptor. In the original Hauser publication ethyl 2-carboxybenzyl phenyl sulfoxide reacts with 3-pentene-2-one with LDA
as a base in THF at -78°C :
 The original reaction product still contains the sulfoxide group but it is lost on heating in an elimination reaction. The ultimate reaction product is a naphthalene
The original reaction product still contains the sulfoxide group but it is lost on heating in an elimination reaction. The ultimate reaction product is a naphthalene
derivative. The dual purpose of the sulfoxide group is as stabilizing group for the carbanion
in the first reaction step and as leaving group
in the second. This use of sulfoxide is similar to its role in the various Julia coupling reactions.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
used to create a six-member ring α,β-unsaturated cyclic ketone, using a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
(or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
) and methyl vinyl ketone
Methyl vinyl ketone
Methyl vinyl ketone is a reactive organic compound classified as an enone. It is a colorless, flammable, highly toxic liquid with a pungent odor...
. It is named after Sir Robert Robinson, the British chemist who discovered it while he was at the University of Oxford
University of Oxford
The University of Oxford is a university located in Oxford, United Kingdom. It is the second-oldest surviving university in the world and the oldest in the English-speaking world. Although its exact date of foundation is unclear, there is evidence of teaching as far back as 1096...
.

Isoxazole
Isoxazole is an azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring.Isoxazole rings are found in some natural products, such as ibotenic acid. Isoxazoles also form the basis for a number of drugs, including the COX-2 inhibitor valdecoxib...
s will give the same product.
The Wieland-Miescher ketone
Wieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
is the Robinson annulation product of 2-methyl-1,3-cyclohexanedione and methyl vinyl ketone
Methyl vinyl ketone
Methyl vinyl ketone is a reactive organic compound classified as an enone. It is a colorless, flammable, highly toxic liquid with a pungent odor...
while the Hajos-Parrish ketone is the product of 2-methyl-1,3-cyclopentanedione and methyl vinyl ketone
Methyl vinyl ketone
Methyl vinyl ketone is a reactive organic compound classified as an enone. It is a colorless, flammable, highly toxic liquid with a pungent odor...
. Asymmetric synthesis of these compounds has greatly increased their synthetic utility.
Reaction mechanism
Methyl vinyl ketone (or variants thereof) are essential for the annulationAnnulation
Annulation in organic chemistry is a chemical reaction in which a new ring is constructed on another molecule ....
as they are simultaneously a Michael acceptor and able to take part in an aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
. The first step in the Robinson annulation (also spelt annelation) is a Michael addition
Michael reaction
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
followed by an aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
as the annulation
Annulation
Annulation in organic chemistry is a chemical reaction in which a new ring is constructed on another molecule ....
step in the process. The reaction then proceeds as an aldol condensation to make the desired cyclohexenone
Cyclohexenone
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances...
ring.

Asymmetric Robinson annulation
The organocatalyst ProlineProline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
has been used to resolve the enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
ic isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s of Robinson annulation
Annulation
Annulation in organic chemistry is a chemical reaction in which a new ring is constructed on another molecule ....
s in asymmetric synthesis. A proline derivative was employed in an asymmetric annulation of a geranial :

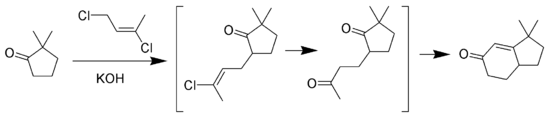
Wichterle reaction
The Wichterle reaction is a variant of the Robinson annulation that replaces methyl vinyl ketone with 1,3-dichloro-cis-2-butene.
Hauser annulation
The reaction sequence in the related Hauser annulation is Michael addition - Dieckman condensation - eliminationElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
. The Hauser donor is an aromatic methylene sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
or sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
with a carboxylic ester group in the ortho position
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
. The Hauser acceptor is a Michael acceptor. In the original Hauser publication ethyl 2-carboxybenzyl phenyl sulfoxide reacts with 3-pentene-2-one with LDA
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
as a base in THF at -78°C :

Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
derivative. The dual purpose of the sulfoxide group is as stabilizing group for the carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
in the first reaction step and as leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
in the second. This use of sulfoxide is similar to its role in the various Julia coupling reactions.

