Reduction of nitro compounds
Encyclopedia
The chemical reaction
s described as reduction of nitro compounds can be facilitated by many different reagents and reaction conditions. Historically, the nitro group
was one of the first functional group
s to be reduced
, due to the ease of nitro-group reduction.
Nitro-groups behave differently whether a neighboring hydrogen
is present or not. Thus, reduction conditions can be initially classified by starting materials: aliphatic nitro compounds or aromatic nitro compounds. Secondary classifications are based upon reaction products.
Reduction to hydrocarbon
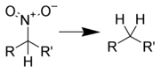
 Hydrodenitration (replacement of a nitro group with hydrogen
Hydrodenitration (replacement of a nitro group with hydrogen
) is difficult to achieve, but can be completed by catalytic hydrogenation over platinum
on silica gel
at high temperatures.
Reduction to amine
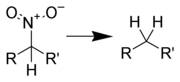
 Aliphatic nitro compounds can be reduced to aliphatic amines using several different reagents:
Aliphatic nitro compounds can be reduced to aliphatic amines using several different reagents:
α,β-Unsaturated nitro compounds can be reduced to saturated amines using:
Reduction to hydroxylamine
 Aliphatic nitro compounds can be reduced to aliphatic hydroxylamines using diborane
Aliphatic nitro compounds can be reduced to aliphatic hydroxylamines using diborane
.
Reduction to oxime
 Nitro compounds are typically reduced to oximes using metal salts, such as stannous chloride or chromium(II) chloride
Nitro compounds are typically reduced to oximes using metal salts, such as stannous chloride or chromium(II) chloride
. Additionally, catalytic hydrogenation using a controlled amount of hydrogen can generate oximes.
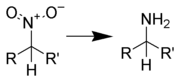
Reduction to aniline
 Many methods for the production of anilines from aryl nitro compounds exist, such as:
Many methods for the production of anilines from aryl nitro compounds exist, such as:
It is also possible to form a nitroaniline
by reduction of a dinitroarene using sodium sulfide
.
Metal hydrides are typically not used to reduce aryl nitro compounds to anilines because they tend to produce azo compounds. (See below)
Reduction to hydroxylamine
Several methods for the production of aryl hydroxylamines from aryl nitro compounds exist:
Reduction to hydrazo compound
Treatment of nitroarenes with excess zinc
metal results in the formation of N,N-diarylhydrazine.
Reduction to azo compound
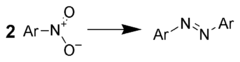 Treatment of aromatic nitro compounds with metal hydrides gives good yields of azo compound
Treatment of aromatic nitro compounds with metal hydrides gives good yields of azo compound
s. For example, one could use:
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s described as reduction of nitro compounds can be facilitated by many different reagents and reaction conditions. Historically, the nitro group
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
was one of the first functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s to be reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, due to the ease of nitro-group reduction.
Nitro-groups behave differently whether a neighboring hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
is present or not. Thus, reduction conditions can be initially classified by starting materials: aliphatic nitro compounds or aromatic nitro compounds. Secondary classifications are based upon reaction products.
Reduction to hydrocarbonHydrocarbonIn organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s

Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
) is difficult to achieve, but can be completed by catalytic hydrogenation over platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
on silica gel
Silica gel
Silica gel is a granular, vitreous, porous form of silica made synthetically from sodium silicate. Despite its name, silica gel is a solid. It is a naturally occurring mineral that is purified and processed into either granular or beaded form...
at high temperatures.
Reduction to amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s

- Catalytic hydrogenation using platinum(IV) oxide (PtO2) or Raney nickelRaney nickelRaney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
- IronIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
metal in refluxing acetic acidAcetic acidAcetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell... - Samarium diiodideSamarium(II) iodideSamarium iodide is a green solid composed of samarium and iodine, with a melting point of 520 °C where the samarium atom has a coordination number of seven in a capped octahedral configuration...
α,β-Unsaturated nitro compounds can be reduced to saturated amines using:
- Catalytic hydrogenation over palladium-on-carbon
- Iron metal
- Lithium aluminium hydrideLithium aluminium hydrideLithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
(Note: Hydroxylamine and oxime impurities are typically found.)
Reduction to hydroxylamineHydroxylamineHydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
s

Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
.
Reduction to oximeOximeAn oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s

Chromium(II) chloride
Chromium chloride is the chemical compound with the formula CrCl2. This white, crystalline solid is used for the synthesis of other chromium complexes. CrCl2 is hygroscopic. It dissolves in water to give bright blue solutions that are easily oxidized by air to give Cr-containing products...
. Additionally, catalytic hydrogenation using a controlled amount of hydrogen can generate oximes.
Aromatic nitro compounds
The reduction of aryl nitro compounds can be finely tuned to obtain a different products typically in high yields.Reduction to anilineAnilineAniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s

- Catalytic hydrogenation using palladium-on-carbon, platinum(IV) oxide, or Raney nickelRaney nickelRaney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
- IronIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
in acidic media (Note: Iron is particularly well suited for this reduction as the reaction conditions are typically gentle and also because iron has a high functional group tolerance.) (See Bechamp reductionBechamp ReductionThe Bechamp reduction is used to reduce aromatic nitro compounds to their corresponding anilines, using iron and hydrochloric acid.This reaction was originally used to produce large amounts of aniline for industry, but catalytic hydrogenation is the preferred method...
) - Sodium hydrosulfite
- Sodium sulfideSodium sulfideSodium sulfide is the name used to refer to the chemical compound Na2S, but more commonly it refers to the hydrate Na2S·9H2O. Both are colorless water-soluble salts that give strongly alkaline solutions...
(or hydrogen sulfideHydrogen sulfideHydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and base) - Tin(II) chlorideTin(II) chlorideTin chloride is a white crystalline solid with the formula 2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent , and in electrolytic baths for tin-plating...
- Titanium(III) chlorideTitanium(III) chlorideTitanium chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known...
- ZincZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
- SamariumSamariumSamarium is a chemical element with the symbol Sm, atomic number 62 and atomic weight 150.36. It is a moderately hard silvery metal which readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3...
It is also possible to form a nitroaniline
Nitroaniline
The term nitroaniline in chemistry refers to a derivative of aniline containing a nitro group There are three simple nitroanilines of formula C6H4 which differ only in the position of the nitro group:* 2-Nitroaniline* 3-Nitroaniline...
by reduction of a dinitroarene using sodium sulfide
Sodium sulfide
Sodium sulfide is the name used to refer to the chemical compound Na2S, but more commonly it refers to the hydrate Na2S·9H2O. Both are colorless water-soluble salts that give strongly alkaline solutions...
.
Metal hydrides are typically not used to reduce aryl nitro compounds to anilines because they tend to produce azo compounds. (See below)
Reduction to hydroxylamineHydroxylamineHydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
s
Several methods for the production of aryl hydroxylamines from aryl nitro compounds exist:
- Raney nickelRaney nickelRaney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
and hydrazineHydrazineHydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
at 0-10 °C - Electrolytic reduction
- ZincZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
metal in aqueous ammonium chloride
Reduction to hydrazo compoundHydrazineHydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
s
Treatment of nitroarenes with excess zincZinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
metal results in the formation of N,N-diarylhydrazine.
Reduction to azo compoundAzo compoundAzo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
s

Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
s. For example, one could use:
- Lithium aluminium hydrideLithium aluminium hydrideLithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
- ZincZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
metal with sodium hydroxide. (Excess zinc will reduce the azo group to a hydrazino compound.)

