
Bell–Evans–Polanyi principle
Encyclopedia
In physical chemistry
, the Bell–Evans–Polanyi principle observes that in a series of similar homolytic atom transfer reactions of the general type, there is often a closely linear relationship between the activation energy
and the reaction enthalpy. This can be expressed as:
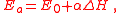
where:
In terms of reaction energy diagrams, this indicates that for reactions having similar energy profiles, the height of the barrier will decrease as the reaction become more exothermic
.
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
, the Bell–Evans–Polanyi principle observes that in a series of similar homolytic atom transfer reactions of the general type, there is often a closely linear relationship between the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and the reaction enthalpy. This can be expressed as:
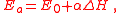
where:
- E0 is the activation energy of the reference reaction
- ΔH is the enthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of each reaction in the series
In terms of reaction energy diagrams, this indicates that for reactions having similar energy profiles, the height of the barrier will decrease as the reaction become more exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
.

