
Reactions on surfaces
Encyclopedia
By reactions on surfaces it is understood reactions in which at least one of the steps of the reaction mechanism
is the adsorption
of one or more reactants. The mechanisms for these reactions, and the rate equation
s are of extreme importance for heterogeneous catalysis
.
A + S AS → Products
Where A is the reactant and S is an adsorption site on the surface. If the rate constants for the adsorption, desorption and reaction are k1, k-1 and k2 , then the global reaction rate is: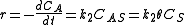
where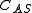 is the concentration of occupied sites,
is the concentration of occupied sites, 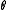 is the surface coverage and
is the surface coverage and  is the total number of sites (occupied or not).
is the total number of sites (occupied or not).
 is highly related to the total surface area of the adsorbent: the bigger the surface area, the more sites and the faster the reaction. This is the reason why heterogeneous catalysts are usually chosen to have great surface areas (in the order of a hundred m2/gram)
is highly related to the total surface area of the adsorbent: the bigger the surface area, the more sites and the faster the reaction. This is the reason why heterogeneous catalysts are usually chosen to have great surface areas (in the order of a hundred m2/gram)
If we apply the steady state
approximation to AS, then
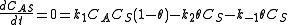 so
so 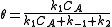 and
and  . Please notice that, with
. Please notice that, with 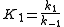 , the formula was divided by
, the formula was divided by 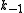 .
.
The result is completely equivalent to the Michaelis-Menten kinetics
. The rate equation is complex, and the reaction order is not clear. In experimental work, usually two extreme cases are looked for in order to prove the mechanism. In them, the rate-determining step
can be:
 , so
, so 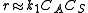 .
.
The order respect to A is 1. Examples of this mechanism are N2O
on gold and HI on platinum
 so
so 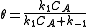 which is just Langmuir isotherm
which is just Langmuir isotherm
and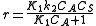 . Depending on the concentration of the reactant the rate changes:
. Depending on the concentration of the reactant the rate changes:
Langmuir
Langmuir-Heishelwood-Hougen-Watson
This mechanism proposes that both molecules adsorb and the adsorbed molecules undergo a bimolecular reaction:
A + S AS
B + S BS
AS + BS → Products
The rate constants are now ,
, ,
, ,
,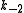 and
and  for adsorption/desorption of A, adsorption/desorption of B, and reaction. The rate law is:
for adsorption/desorption of A, adsorption/desorption of B, and reaction. The rate law is: 
Proceeding as before we get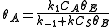 , where
, where 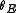 is the fraction of empty sites, so
is the fraction of empty sites, so 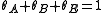 . Let us assume now that the rate limiting step is the reaction of the adsorbed molecules, which is easily understood: the probability of two adsorbed molecules colliding is low.
. Let us assume now that the rate limiting step is the reaction of the adsorbed molecules, which is easily understood: the probability of two adsorbed molecules colliding is low.
Then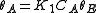 , with
, with  , which is nothing but Langmuir isotherm for two adsorbed gases, with adsorption constants
, which is nothing but Langmuir isotherm for two adsorbed gases, with adsorption constants  and
and 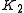 .
.
Calculating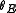 from
from 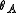 and
and 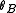 we finally get
we finally get
The rate law is complex and there is no clear order respect to any of the reactants but we can consider different values of the constants, for which it is easy to measure integer orders:
That means that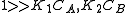 , so
, so 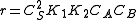 . The order is one respect to both the reactants
. The order is one respect to both the reactants
In this case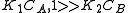 , so
, so 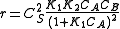 . The reaction order is 1 respect to B. There are two extreme possibilities now:
. The reaction order is 1 respect to B. There are two extreme possibilities now:
One of the reactants has very high adsorption and the other one doesn't adsorb strongly.
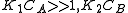 , so
, so  . The reaction order is 1 respect to B and -1 respect to A. Reactant A inhibits the reaction at all concentrations.
. The reaction order is 1 respect to B and -1 respect to A. Reactant A inhibits the reaction at all concentrations.
The following reactions follow a Langmuir-Hinshelwood mechanism http://www.theochem.uni-duisburg.de/DC/material/exarbeiten/Exarbeit-Alex/a_7.htm:
A(g) + S(s) AS(s)
AS(s) + B(g) → Products
Constants are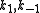 and
and  and rate equation is
and rate equation is  . Applying steady state approximation to AS and proceeding as before (considering the reaction the limiting step once more) we get
. Applying steady state approximation to AS and proceeding as before (considering the reaction the limiting step once more) we get 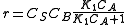 . The order is one respect to B. There are two possibilities, depending on the concentration of reactant A:
. The order is one respect to B. There are two possibilities, depending on the concentration of reactant A:
The following reactions follow a Eley-Rideal mechanism http://www.theochem.uni-duisburg.de/DC/material/exarbeiten/Exarbeit-Alex/a_7.htm:
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is the adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
of one or more reactants. The mechanisms for these reactions, and the rate equation
Rate equation
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant parameters . To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system...
s are of extreme importance for heterogeneous catalysis
Heterogeneous catalysis
In chemistry, heterogeneous catalysis refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but also immiscible liquids, e.g. oil and water. The great majority of practical heterogeneous catalysts...
.
Simple decomposition
If a reaction occurs through these steps:A + S AS → Products
Where A is the reactant and S is an adsorption site on the surface. If the rate constants for the adsorption, desorption and reaction are k1, k-1 and k2 , then the global reaction rate is:
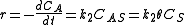
where
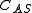 is the concentration of occupied sites,
is the concentration of occupied sites, 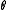 is the surface coverage and
is the surface coverage and  is the total number of sites (occupied or not).
is the total number of sites (occupied or not). is highly related to the total surface area of the adsorbent: the bigger the surface area, the more sites and the faster the reaction. This is the reason why heterogeneous catalysts are usually chosen to have great surface areas (in the order of a hundred m2/gram)
is highly related to the total surface area of the adsorbent: the bigger the surface area, the more sites and the faster the reaction. This is the reason why heterogeneous catalysts are usually chosen to have great surface areas (in the order of a hundred m2/gram)If we apply the steady state
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
approximation to AS, then
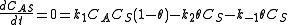 so
so 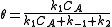 and
and  . Please notice that, with
. Please notice that, with 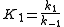 , the formula was divided by
, the formula was divided by 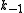 .
.The result is completely equivalent to the Michaelis-Menten kinetics
Michaelis-Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
. The rate equation is complex, and the reaction order is not clear. In experimental work, usually two extreme cases are looked for in order to prove the mechanism. In them, the rate-determining step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
can be:
- Limiting step: Adsorption/Desorption
 , so
, so 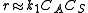 .
.The order respect to A is 1. Examples of this mechanism are N2O
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
on gold and HI on platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
- Limiting Step: Reaction
 so
so 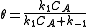 which is just Langmuir isotherm
which is just Langmuir isothermLangmuir equation
The Langmuir equation relates the coverage or adsorption of molecules on a solid surface to gas pressure or concentration of a medium above the solid surface at a fixed temperature. The equation was developed by Irving Langmuir in 1916...
and
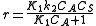 . Depending on the concentration of the reactant the rate changes:
. Depending on the concentration of the reactant the rate changes:
- Low concentrations, then
 , that is to say a first order reaction in component A.
, that is to say a first order reaction in component A. - High concentration, then
 . It is a zeroth order reaction in component A.
. It is a zeroth order reaction in component A.
LangmuirIrving LangmuirIrving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his...
-HinshelwoodCyril Norman HinshelwoodSir Cyril Norman Hinshelwood OM PRS was an English physical chemist.Born in London, his parents were Norman Macmillan Hinshelwood, a chartered accountant, and Ethe Frances née Smith. He was educated first in Canada, returning in 1905 on the death of his father to a small flat in Chelsea where he...
mechanism
Langmuir-Heishelwood-Hougen-WatsonThis mechanism proposes that both molecules adsorb and the adsorbed molecules undergo a bimolecular reaction:
A + S AS
B + S BS
AS + BS → Products
The rate constants are now
 ,
, ,
, ,
,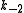 and
and  for adsorption/desorption of A, adsorption/desorption of B, and reaction. The rate law is:
for adsorption/desorption of A, adsorption/desorption of B, and reaction. The rate law is: 
Proceeding as before we get
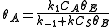 , where
, where 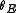 is the fraction of empty sites, so
is the fraction of empty sites, so 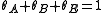 . Let us assume now that the rate limiting step is the reaction of the adsorbed molecules, which is easily understood: the probability of two adsorbed molecules colliding is low.
. Let us assume now that the rate limiting step is the reaction of the adsorbed molecules, which is easily understood: the probability of two adsorbed molecules colliding is low.Then
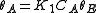 , with
, with  , which is nothing but Langmuir isotherm for two adsorbed gases, with adsorption constants
, which is nothing but Langmuir isotherm for two adsorbed gases, with adsorption constants  and
and 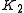 .
.Calculating
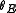 from
from 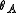 and
and 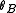 we finally get
we finally get
-
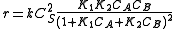 .
.
The rate law is complex and there is no clear order respect to any of the reactants but we can consider different values of the constants, for which it is easy to measure integer orders:
- Both molecules have low adsorption
That means that
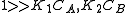 , so
, so 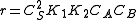 . The order is one respect to both the reactants
. The order is one respect to both the reactants- One molecule has very low adsorption
In this case
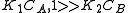 , so
, so 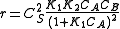 . The reaction order is 1 respect to B. There are two extreme possibilities now:
. The reaction order is 1 respect to B. There are two extreme possibilities now:
-
- At low concentrations of A,
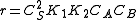 , and the order is one respect to A.
, and the order is one respect to A. - At high concentrations,
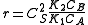 . The order is minus one respect to A. The higher the concentration of A, the slower the reaction goes, in this case we say that A inhibits the reaction.
. The order is minus one respect to A. The higher the concentration of A, the slower the reaction goes, in this case we say that A inhibits the reaction.
- At low concentrations of A,
- One molecule has very high adsorption
One of the reactants has very high adsorption and the other one doesn't adsorb strongly.
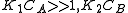 , so
, so  . The reaction order is 1 respect to B and -1 respect to A. Reactant A inhibits the reaction at all concentrations.
. The reaction order is 1 respect to B and -1 respect to A. Reactant A inhibits the reaction at all concentrations.The following reactions follow a Langmuir-Hinshelwood mechanism http://www.theochem.uni-duisburg.de/DC/material/exarbeiten/Exarbeit-Alex/a_7.htm:
- 2 COCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
+ O2OxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
→ 2 CO2Carbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
on a platinumPlatinumPlatinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
catalyst. - CO + 2H2HydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
→ CH3OHMethanolMethanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
on a ZnO catalyst. - C2H4EthyleneEthylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
+ H2 → C2H6EthaneEthane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
on a copperCopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
catalyst. - N2ONitrous oxideNitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
+ H2 → N2NitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
+ H2O on a platinum catalyst. - C2H4 + ½ O2 → CH3CHO on a palladiumPalladiumPalladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalyst. - CO + OH → CO2 + H+ + e- on a platinum catalyst.
Eley-Rideal mechanism
In this mechanism, proposed in 1938 by D. D. Eley and E. K. Rideal, only one of the molecules adsorbs and the other one reacts with it directly from the gas phase, without adsorbing:A(g) + S(s) AS(s)
AS(s) + B(g) → Products
Constants are
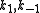 and
and  and rate equation is
and rate equation is  . Applying steady state approximation to AS and proceeding as before (considering the reaction the limiting step once more) we get
. Applying steady state approximation to AS and proceeding as before (considering the reaction the limiting step once more) we get 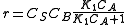 . The order is one respect to B. There are two possibilities, depending on the concentration of reactant A:
. The order is one respect to B. There are two possibilities, depending on the concentration of reactant A:- At low concentrations of A,
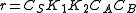 , and the order is one with respect to A.
, and the order is one with respect to A.
- At high concentrations of A,
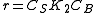 , and the order is zero with respect to A.
, and the order is zero with respect to A.
The following reactions follow a Eley-Rideal mechanism http://www.theochem.uni-duisburg.de/DC/material/exarbeiten/Exarbeit-Alex/a_7.htm:
- C2H4 + ½ O2 (adsorbed) → H2COCH2Ethylene oxideEthylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
The dissociative adsorption of oxygen is also possible, which leads to secondary products carbon dioxide and waterWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. - CO2 + H2(ads.) → H2O + CO
- 2NH3AmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
+ 1½ O2 (ads.) → N2 + 3H2O on a platinum catalyst - C2H2AcetyleneAcetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
+ H2 (ads.) → C2H4 on nickelNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
or ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
catalysts

