Random coil
Encyclopedia
A random coil is a polymer
conformation
where the monomer
subunits are oriented randomly
while still being bonded
to adjacent
units. It is not one specific shape
, but a statistical
distribution of shapes for all the chains in a population
of macromolecule
s. The conformation's name is derived from the idea that, in the absence of specific, stabilizing interactions, a polymer backbone will "sample" all possible conformations randomly. Many linear, unbranched homopolymers — in solution, or above their melting temperatures — assume (approximate
) random coils. Even copolymers with monomers of unequal length
will distribute in random coils if the subunits lack any specific interactions. The parts of branched polymers may also assume random coils.
Below their melting temperatures, most thermoplastic
polymers (polyethylene
, nylon
, etc.) have amorphous
regions in which the chains approximate random coils, alternating with regions that are crystal
line. The amorphous regions contribute elasticity and the crystalline regions contribute strength and rigidity.
More complex polymers such as protein
s, with various interacting chemical groups attached to their backbones, self-assemble
into well-defined structures. But segments of proteins, and polypeptides
that lack secondary structure
, are often assumed to exhibit a random-coil conformation in which the only fixed relationship is the joining of adjacent amino acid
residue
s by a peptide bond
. This is not actually the case, since the ensemble
will be energy
weighted due to interactions between amino acid side-chains, with lower-energy conformations being present more frequently. In addition, even arbitrary sequences of amino acids tend to exhibit some hydrogen bond
ing and secondary structure. For this reason, the term "statistical coil" is occasionally preferred. The conformational entropy
associated with the random-coil state significantly contributes to its energetic stabilization and accounts for much of the energy barrier to protein folding
.
A random-coil conformation can be detected using spectroscopic techniques. The arrangement of the planar amide bonds results in a distinctive signal in circular dichroism
. The chemical shift
of amino acids in a random-coil conformation is well known in nuclear magnetic resonance (NMR). Deviations from these signatures often indicates the presence of some secondary structure, rather than complete random coil. Furthermore, there are signals in multidimensional NMR experiments that indicate that stable, non-local amino acid interactions are absent for polypeptides in a random-coil conformation. Likewise, in the images produced by crystallography
experiments, segments of random coil result simply in a reduction in "electron density" or contrast. A randomly coiled state for any polypeptide chain can be attained by denaturing
the system. However, there is evidence that proteins are never truly random coils, even when denatured (Shortle & Ackerman).
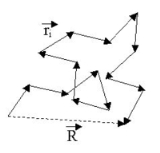
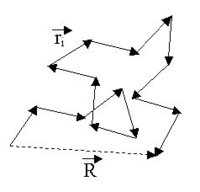 There are an enormous number of different ways
There are an enormous number of different ways
in which a chain can be curled around in a relatively compact shape, like an unraveling ball of twine with lots of open space
, and comparatively few ways it can be more or less stretched out. So, if each conformation has an equal probability
or statistical
weight, chains are much more likely to be ball-like than they are to be extended — a purely entropic
effect. In an ensemble
of chains, most of them will, therefore, be loosely balled up
. This is the kind of shape any one of them will have most of the time.
Consider a linear polymer to be a freely-jointed chain with N subunits, each of length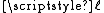 , that occupy zero
, that occupy zero
volume
, so that no part of the chain excludes another from any location. One can regard the segments of each such chain in an ensemble as performing a random walk
(or "random flight") in three dimension
s, limited only by the constraint that each segment must be joined to its neighbors. This is the ideal chain
mathematical model
. It is clear that the maximum, fully extended length L of the chain is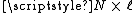 . If we assume that each possible chain conformation has an equal statistical weight, it can be shown
. If we assume that each possible chain conformation has an equal statistical weight, it can be shown
that the probability P(r) of a polymer chain in the population
to have distance r between the ends will obey a characteristic distribution
described by the formula
The average (root mean square
) end-to-end distance for the chain,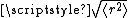 , turns out to be
, turns out to be 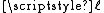 times the square root of N — in other words, the average distance scales with N0.5.
times the square root of N — in other words, the average distance scales with N0.5.
Note that although this model is termed a "Gaussian chain", the distribution function is not a gaussian (normal) distribution. The end-to-end distance probability distribution function of a Gaussian chain is non-zero only for r > 0.
has a fixed tetrahedral angle of 109.5 degrees. The value of L is well-defined for, say, a fully extended polyethylene
or nylon
, but it is less than N x l because of the zig-zag backbone. There is, however, free rotation about many chain bonds. The model above can be enhanced. A longer, "effective" unit length can be defined such that the chain can be regarded as freely-jointed, along with a smaller N, such that the constraint L = N x l is still obeyed. It, too, gives a Gaussian distribution. However, specific cases can also be precisely calculated. The average end-to-end distance for freely-rotating (not freely-jointed) polymethylene (polyethylene with each -C-C- considered as a subunit) is l times the square root of 2N, an increase by a factor of about 1.4. Unlike the zero volume assumed in a random walk calculation, all real polymers' segments occupy space because of the van der Waals radii
of their atoms, including bulky substituent groups
that interfere with bond rotations
. This can also be taken into account in calculations. All such effects increase the mean end-to-end distance.
Because their polymerization is stochastic
ally driven, chain lengths in any real population of synthetic
polymers will obey a statistical distribution. In that case, we should take N to be an average value. Also, many polymers have random branching.
Even with corrections for local constraints, the random walk model ignores steric interference between chains, and between distal parts of the same chain. A chain often cannot move from a given conformation to a closely related one by a small displacement because one part of it would have to pass through another part, or through a neighbor. We may still hope that the ideal-chain, random-coil model will be at least a qualitative indication of the shapes and dimension
s of real polymers in solution
, and in the amorphous state, as long as there are only weak physicochemical interactions
between the monomers. This model, and the Flory-Huggins Solution Theory
, for which Paul Flory
received the Nobel Prize in Chemistry
in 1974, ostensibly apply only to ideal, dilute solutions
. But there is reason to believe (e.g., neutron diffraction
studies) that excluded volume effects
may cancel out, so that, under certain conditions, chain dimensions in amorphous polymers have approximately the ideal, calculated size
When separate chains interact cooperatively, as in forming crystalline regions in solid
thermoplastics, a different mathematical approach must be used.
Stiffer polymers such as helical
polypeptides, Kevlar
, and double-stranded DNA
can be treated by the worm-like chain
model.
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
conformation
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
where the monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
subunits are oriented randomly
Randomness
Randomness has somewhat differing meanings as used in various fields. It also has common meanings which are connected to the notion of predictability of events....
while still being bonded
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
to adjacent
Graph (mathematics)
In mathematics, a graph is an abstract representation of a set of objects where some pairs of the objects are connected by links. The interconnected objects are represented by mathematical abstractions called vertices, and the links that connect some pairs of vertices are called edges...
units. It is not one specific shape
Shape
The shape of an object located in some space is a geometrical description of the part of that space occupied by the object, as determined by its external boundary – abstracting from location and orientation in space, size, and other properties such as colour, content, and material...
, but a statistical
Statistics
Statistics is the study of the collection, organization, analysis, and interpretation of data. It deals with all aspects of this, including the planning of data collection in terms of the design of surveys and experiments....
distribution of shapes for all the chains in a population
Statistical population
A statistical population is a set of entities concerning which statistical inferences are to be drawn, often based on a random sample taken from the population. For example, if we were interested in generalizations about crows, then we would describe the set of crows that is of interest...
of macromolecule
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
s. The conformation's name is derived from the idea that, in the absence of specific, stabilizing interactions, a polymer backbone will "sample" all possible conformations randomly. Many linear, unbranched homopolymers — in solution, or above their melting temperatures — assume (approximate
Approximation
An approximation is a representation of something that is not exact, but still close enough to be useful. Although approximation is most often applied to numbers, it is also frequently applied to such things as mathematical functions, shapes, and physical laws.Approximations may be used because...
) random coils. Even copolymers with monomers of unequal length
Length
In geometric measurements, length most commonly refers to the longest dimension of an object.In certain contexts, the term "length" is reserved for a certain dimension of an object along which the length is measured. For example it is possible to cut a length of a wire which is shorter than wire...
will distribute in random coils if the subunits lack any specific interactions. The parts of branched polymers may also assume random coils.
Below their melting temperatures, most thermoplastic
Thermoplastic
Thermoplastic, also known as a thermosoftening plastic, is a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently...
polymers (polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
, nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
, etc.) have amorphous
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
regions in which the chains approximate random coils, alternating with regions that are crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line. The amorphous regions contribute elasticity and the crystalline regions contribute strength and rigidity.
More complex polymers such as protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, with various interacting chemical groups attached to their backbones, self-assemble
Molecular self-assembly
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly, intramolecular self-assembly and intermolecular self-assembly...
into well-defined structures. But segments of proteins, and polypeptides
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
that lack secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
, are often assumed to exhibit a random-coil conformation in which the only fixed relationship is the joining of adjacent amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
s by a peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
. This is not actually the case, since the ensemble
Statistical ensemble (mathematical physics)
In mathematical physics, especially as introduced into statistical mechanics and thermodynamics by J. Willard Gibbs in 1878, an ensemble is an idealization consisting of a large number of mental copies of a system, considered all at once, each of which represents a possible state that the real...
will be energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
weighted due to interactions between amino acid side-chains, with lower-energy conformations being present more frequently. In addition, even arbitrary sequences of amino acids tend to exhibit some hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing and secondary structure. For this reason, the term "statistical coil" is occasionally preferred. The conformational entropy
Conformational entropy
Conformational entropy is the entropy associated with the physical arrangement of a polymer chain that assumes a compact or globular state in solution. The concept is most commonly applied to biological macromolecules such as proteins and RNA, but can also be used for polysaccharides and other...
associated with the random-coil state significantly contributes to its energetic stabilization and accounts for much of the energy barrier to protein folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
.
A random-coil conformation can be detected using spectroscopic techniques. The arrangement of the planar amide bonds results in a distinctive signal in circular dichroism
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
. The chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
of amino acids in a random-coil conformation is well known in nuclear magnetic resonance (NMR). Deviations from these signatures often indicates the presence of some secondary structure, rather than complete random coil. Furthermore, there are signals in multidimensional NMR experiments that indicate that stable, non-local amino acid interactions are absent for polypeptides in a random-coil conformation. Likewise, in the images produced by crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
experiments, segments of random coil result simply in a reduction in "electron density" or contrast. A randomly coiled state for any polypeptide chain can be attained by denaturing
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
the system. However, there is evidence that proteins are never truly random coils, even when denatured (Shortle & Ackerman).
Random walk model: The Gaussian chain

Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
in which a chain can be curled around in a relatively compact shape, like an unraveling ball of twine with lots of open space
Space
Space is the boundless, three-dimensional extent in which objects and events occur and have relative position and direction. Physical space is often conceived in three linear dimensions, although modern physicists usually consider it, with time, to be part of a boundless four-dimensional continuum...
, and comparatively few ways it can be more or less stretched out. So, if each conformation has an equal probability
Probability
Probability is ordinarily used to describe an attitude of mind towards some proposition of whose truth we arenot certain. The proposition of interest is usually of the form "Will a specific event occur?" The attitude of mind is of the form "How certain are we that the event will occur?" The...
or statistical
Statistics
Statistics is the study of the collection, organization, analysis, and interpretation of data. It deals with all aspects of this, including the planning of data collection in terms of the design of surveys and experiments....
weight, chains are much more likely to be ball-like than they are to be extended — a purely entropic
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
effect. In an ensemble
Statistical ensemble (mathematical physics)
In mathematical physics, especially as introduced into statistical mechanics and thermodynamics by J. Willard Gibbs in 1878, an ensemble is an idealization consisting of a large number of mental copies of a system, considered all at once, each of which represents a possible state that the real...
of chains, most of them will, therefore, be loosely balled up
Sphere
A sphere is a perfectly round geometrical object in three-dimensional space, such as the shape of a round ball. Like a circle in two dimensions, a perfect sphere is completely symmetrical around its center, with all points on the surface lying the same distance r from the center point...
. This is the kind of shape any one of them will have most of the time.
Consider a linear polymer to be a freely-jointed chain with N subunits, each of length
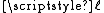 , that occupy zero
, that occupy zero0 (number)
0 is both a numberand the numerical digit used to represent that number in numerals.It fulfills a central role in mathematics as the additive identity of the integers, real numbers, and many other algebraic structures. As a digit, 0 is used as a placeholder in place value systems...
volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
, so that no part of the chain excludes another from any location. One can regard the segments of each such chain in an ensemble as performing a random walk
Random walk
A random walk, sometimes denoted RW, is a mathematical formalisation of a trajectory that consists of taking successive random steps. For example, the path traced by a molecule as it travels in a liquid or a gas, the search path of a foraging animal, the price of a fluctuating stock and the...
(or "random flight") in three dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
s, limited only by the constraint that each segment must be joined to its neighbors. This is the ideal chain
Ideal chain
An ideal chain is the simplest model to describe a polymer. It only assumes a polymer as a random walk and neglects any kind of interactions among monomers...
mathematical model
Mathematical model
A mathematical model is a description of a system using mathematical concepts and language. The process of developing a mathematical model is termed mathematical modeling. Mathematical models are used not only in the natural sciences and engineering disciplines A mathematical model is a...
. It is clear that the maximum, fully extended length L of the chain is
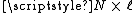 . If we assume that each possible chain conformation has an equal statistical weight, it can be shown
. If we assume that each possible chain conformation has an equal statistical weight, it can be shownIdeal chain
An ideal chain is the simplest model to describe a polymer. It only assumes a polymer as a random walk and neglects any kind of interactions among monomers...
that the probability P(r) of a polymer chain in the population
Statistical population
A statistical population is a set of entities concerning which statistical inferences are to be drawn, often based on a random sample taken from the population. For example, if we were interested in generalizations about crows, then we would describe the set of crows that is of interest...
to have distance r between the ends will obey a characteristic distribution
Probability distribution
In probability theory, a probability mass, probability density, or probability distribution is a function that describes the probability of a random variable taking certain values....
described by the formula
The average (root mean square
Root mean square
In mathematics, the root mean square , also known as the quadratic mean, is a statistical measure of the magnitude of a varying quantity. It is especially useful when variates are positive and negative, e.g., sinusoids...
) end-to-end distance for the chain,
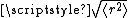 , turns out to be
, turns out to be 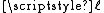 times the square root of N — in other words, the average distance scales with N0.5.
times the square root of N — in other words, the average distance scales with N0.5.Note that although this model is termed a "Gaussian chain", the distribution function is not a gaussian (normal) distribution. The end-to-end distance probability distribution function of a Gaussian chain is non-zero only for r > 0.
Real polymers
A real polymer is not freely-jointed. A -C-C- single bondChemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
has a fixed tetrahedral angle of 109.5 degrees. The value of L is well-defined for, say, a fully extended polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
or nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
, but it is less than N x l because of the zig-zag backbone. There is, however, free rotation about many chain bonds. The model above can be enhanced. A longer, "effective" unit length can be defined such that the chain can be regarded as freely-jointed, along with a smaller N, such that the constraint L = N x l is still obeyed. It, too, gives a Gaussian distribution. However, specific cases can also be precisely calculated. The average end-to-end distance for freely-rotating (not freely-jointed) polymethylene (polyethylene with each -C-C- considered as a subunit) is l times the square root of 2N, an increase by a factor of about 1.4. Unlike the zero volume assumed in a random walk calculation, all real polymers' segments occupy space because of the van der Waals radii
Van der Waals radius
The van der Waals radius, r, of an atom is the radius of an imaginary hard sphere which can be used to model the atom for many purposes. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms had a finite size and to...
of their atoms, including bulky substituent groups
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
that interfere with bond rotations
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
. This can also be taken into account in calculations. All such effects increase the mean end-to-end distance.
Because their polymerization is stochastic
Stochastic
Stochastic refers to systems whose behaviour is intrinsically non-deterministic. A stochastic process is one whose behavior is non-deterministic, in that a system's subsequent state is determined both by the process's predictable actions and by a random element. However, according to M. Kac and E...
ally driven, chain lengths in any real population of synthetic
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
polymers will obey a statistical distribution. In that case, we should take N to be an average value. Also, many polymers have random branching.
Even with corrections for local constraints, the random walk model ignores steric interference between chains, and between distal parts of the same chain. A chain often cannot move from a given conformation to a closely related one by a small displacement because one part of it would have to pass through another part, or through a neighbor. We may still hope that the ideal-chain, random-coil model will be at least a qualitative indication of the shapes and dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
s of real polymers in solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
, and in the amorphous state, as long as there are only weak physicochemical interactions
Intermolecular force
Intermolecular forces are forces of attraction or repulsion which act between neighboring particles: atoms, molecules or ions. They are weak compared to the intramolecular forces, the forces which keep a molecule together...
between the monomers. This model, and the Flory-Huggins Solution Theory
Flory-Huggins solution theory
Flory-Huggins solution theory is a mathematical model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecular sizes in adapting the usual expression for the entropy of mixing. The result is an equation for the Gibbs free energy change \Delta G_m for...
, for which Paul Flory
Paul Flory
Paul John Flory was an American chemist and Nobel laureate who was known for his prodigious volume of work in the field of polymers, or macromolecules...
received the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 1974, ostensibly apply only to ideal, dilute solutions
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
. But there is reason to believe (e.g., neutron diffraction
Neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material: A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of...
studies) that excluded volume effects
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
may cancel out, so that, under certain conditions, chain dimensions in amorphous polymers have approximately the ideal, calculated size
When separate chains interact cooperatively, as in forming crystalline regions in solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
thermoplastics, a different mathematical approach must be used.
Stiffer polymers such as helical
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
polypeptides, Kevlar
Kevlar
Kevlar is the registered trademark for a para-aramid synthetic fiber, related to other aramids such as Nomex and Technora. Developed at DuPont in 1965, this high strength material was first commercially used in the early 1970s as a replacement for steel in racing tires...
, and double-stranded DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
can be treated by the worm-like chain
Worm-like chain
The worm-like chain model in polymer physics is used to describe the behavior of semi-flexible polymers; it is sometimes referred to as the Kratky-Porod model.- Theoretical Considerations :...
model.
External links
- polymer statistical mechanics
- A topological problem in polymer physics: configurational and mechanical properties of a random walk enclosing a constant are
- D. Shortle and M. Ackerman, Persistence of native-like topology in a denatured protein in 8 M urea, Science 293 (2001), pp. 487–489
- Sample chapter "Conformations, Solutions, and Molecular Weight" from "Polymer Science & Technology" courtesy of Prentice Hall Professional publications


