Nylon
Overview
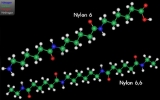
Nylon is a generic designation for a family of synthetic polymer
Synthetic polymer
Synthetic polymers are often referred to as "plastics", such as the well-known polyethylene and nylon. However, most of them can be classified in at least three main categories: thermoplastics, thermosets and elastomers....
s known generically as polyamide
Polyamide
A polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
s, first produced on February 28, 1935, by Wallace Carothers
Wallace Carothers
Wallace Hume Carothers was an American chemist, inventor and the leader of organic chemistry at DuPont, credited with the invention of nylon....
at DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
's research facility at the DuPont Experimental Station
DuPont Experimental Station
The DuPont Experimental Station is the largest research and development facility of E. I. du Pont de Nemours and Company. Located on the banks of the Brandywine Creek in Wilmington, Delaware, it is home to some of the most important discoveries of the modern chemical industry...
. Nylon is one of the most commonly used polymers.
Nylon is a thermoplastic
Thermoplastic
Thermoplastic, also known as a thermosoftening plastic, is a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently...
, silky material, first used commercially in a nylon-bristle
Bristle
A bristle is a stiff hair or feather. Also used are synthetic materials such as nylon in items such as brooms and sweepers. Bristles are often used to make brushes for cleaning uses, as they are strongly abrasive; common examples include the toothbrush and toilet brush...
d toothbrush
Toothbrush
The toothbrush is an oral hygiene instrument used to clean the teeth and gums that consists of a head of tightly clustered bristles mounted on a handle, which facilitates the cleansing of hard-to-reach areas of the mouth. Toothpaste, which often contains fluoride, is commonly used in conjunction...
(1938), followed more famously by women's stocking
Stocking
A stocking, , is a close-fitting, variously elastic garment covering the foot and lower part of the leg. Stockings vary in color, design and transparency...
s ("nylons"; 1940). It is made of repeating units
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
linked by amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
and is frequently referred to as polyamide
Polyamide
A polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
(PA).

