
Radioanalytical chemistry
Encyclopedia
Radioanalytical chemistry focuses on the analysis of sample for their radionuclide
content. Various methods are employed to purify and identify the radioelement of interest through chemical methods and sample measurement techniques.
with contributions by Ernest Rutherford
and Frederick Soddy
. They developed chemical separation and radiation measurement techniques on terrestrial radioactive substances. During the twenty years that followed 1897 the concepts of radionuclides was born. Since Curie's time, applications of radioanalytical chemistry have proliferated. Modern advances in nuclear and radiochemistry research have allowed practitioners to apply chemistry and nuclear procedures to elucidate nuclear properties and reactions, used radioactive substances as tracers, and measure radionuclides in many different types of samples.
The importance of radioanalytical chemistry spans many fields including chemistry
, physics
, medicine
, pharmacology
, biology
, ecology
, hydrology
, geology
, forensics
, atmospheric sciences
, health protection, archeology, and engineering
. Applications include: forming and characterizing new elements, determining the age of materials, and creating radioactive reagents for specific tracer use in tissues and organs. The ongoing goal of radioanalytical researchers is to develop more radionuclides and lower concentrations in people and the environment.
is characterized by the emission of an alpha particle, a 4He nucleus. The mode of this decay causes the parent nucleus to decrease by two protons and two neutrons. This type of decay follows the relation:
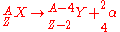
is characterized by the emission of a neutrino
and a negatron which is equivalent to an electron
. This process occurs when a nucleus has an excess of neutrons with respect to protons, as compared to the stable isobar
. This type of transition converts a neutron into a proton; similarly, a positron
is released when a proton is converted into a neutron. These decays follows the relation:
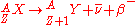
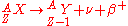
emission is follows the previously discussed modes of decay when the decay leaves a daughter nucleus in an excited state. This nucleus is capable of further de-excitation to a lower energy state by the release of a photon. This decay follows the relation:
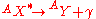
collect and record the electrons freed from gaseous atoms and molecules by the interaction of radiation released by the source. A voltage potential
is applied between two electrodes within a sealed system. Since the gaseous atoms are ionized after they interact with radiation they are attracted to the anode which produces a signal. It is important to vary the applied voltage such that the response falls within a critical proportional range.
s is similar to gas ionization detectors expect instead of ionization gas atoms, free electrons and holes are produced which create a signal at the electrodes. The advantage of solid state detectors is the greater resolution of the resultant energy spectrum. Usually NaI(Tl) detectors are used; for more precise applications Ge(Li) and Si(Li) detectors have been developed. For extra sensitive measurements high-pure germanium detectors are used under a liquid nitrogen environment.
detectors uses a photo luminescent source (such as ZnS) which interacts with radiation. When a radioactive particle decays and strikes the photo luminescent material a photon is released. This photon is multiplied in a photomultiplier tube which converts light into an electrical signal. This signal is then processed and converted into a channel. By comparing the number of counts to the energy level (typically in keV or MeV) the type of decay can be determined.
separation techniques can be used. These separation methods include precipitation
, Ion Exchange
, Liquid Liquid
extraction, Solid
Phase extraction, Distillation
, and Electrodeposition
.
ic or electrostatic adsorption, as well as metal foils and glass slides. Sample loss is an ever present concern, especially at the beginning of the analysis path where sequential steps may compound these losses.
Various solutions are known to circumvent these losses which include adding an inactive carrier
or adding a tracer
. Research has also shown that that pretreatment of glassware and plastic surfaces can reduce radionuclide sorption by saturating the sites.
Carrier addition is the reverse technique of tracer addition. Instead of isotope dilution, a known mass of stable carrier ion is added to radionuclide sample solution. The carrier reagent must be calibrated prior to addition to the sample. To verify the resultant measurements, the expected 100% yield is compared to the actual yield. Any loss in yield is analogous to any losses in the radioactive sample. Typically the amount of carrier added is conventionally selected for the ease of weighing such that the accuracy of the resultant weight is within 1%. For alpha particles, special techniques must be applied to obtain the required thin sample sources.
technique quality control
is an important factor to maintain. A laboratory
must produce trustworthy results. This can be accomplished by a laboratories continual effort to maintain instrument
calibration
, measurement reproducibility, and applicability of analytical methods. In all laboratories there must be a quality assurance plan. This plan describes the quality system and procedures in place to obtain consistent results. Such results must be authentic, appropriately documented, and technically defensible." Such elements of quality assurance include organization, personnel training, laboratory operating procedures, procurement documents, chain of custody records, standard certificates, analytical records, standard procedures, QC sample analysis program and results, instrument testing and maintenance records, results of performance demonstration projects, results of data assessment, audit reports, and record retention policies.
The cost of quality assurance is continually on the rise but the benefits far outweigh this cost. The average quality assurance workload was risen from 10% to a modern load of 20-30%. This heightened focus on quality assurance ensures that quality measurements that are reliable are achieved. The cost of failure far outweighs the cost of prevention and appraisal. Finally, results must be scientifically defensible by adhering to stringent regulations in the event of a lawsuit.
Radionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
content. Various methods are employed to purify and identify the radioelement of interest through chemical methods and sample measurement techniques.
History
The field of radioanalytical chemistry was originally developed by Marie CurieMarie Curie
Marie Skłodowska-Curie was a physicist and chemist famous for her pioneering research on radioactivity. She was the first person honored with two Nobel Prizes—in physics and chemistry...
with contributions by Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
and Frederick Soddy
Frederick Soddy
Frederick Soddy was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also proved the existence of isotopes of certain radioactive elements...
. They developed chemical separation and radiation measurement techniques on terrestrial radioactive substances. During the twenty years that followed 1897 the concepts of radionuclides was born. Since Curie's time, applications of radioanalytical chemistry have proliferated. Modern advances in nuclear and radiochemistry research have allowed practitioners to apply chemistry and nuclear procedures to elucidate nuclear properties and reactions, used radioactive substances as tracers, and measure radionuclides in many different types of samples.
The importance of radioanalytical chemistry spans many fields including chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, medicine
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
, pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
, biology
Biology
Biology is a natural science concerned with the study of life and living organisms, including their structure, function, growth, origin, evolution, distribution, and taxonomy. Biology is a vast subject containing many subdivisions, topics, and disciplines...
, ecology
Ecology
Ecology is the scientific study of the relations that living organisms have with respect to each other and their natural environment. Variables of interest to ecologists include the composition, distribution, amount , number, and changing states of organisms within and among ecosystems...
, hydrology
Hydrology
Hydrology is the study of the movement, distribution, and quality of water on Earth and other planets, including the hydrologic cycle, water resources and environmental watershed sustainability...
, geology
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
, forensics
Forensics
Forensic science is the application of a broad spectrum of sciences to answer questions of interest to a legal system. This may be in relation to a crime or a civil action...
, atmospheric sciences
Atmospheric sciences
Atmospheric sciences is an umbrella term for the study of the atmosphere, its processes, the effects other systems have on the atmosphere, and the effects of the atmosphere on these other systems. Meteorology includes atmospheric chemistry and atmospheric physics with a major focus on weather...
, health protection, archeology, and engineering
Engineering
Engineering is the discipline, art, skill and profession of acquiring and applying scientific, mathematical, economic, social, and practical knowledge, in order to design and build structures, machines, devices, systems, materials and processes that safely realize improvements to the lives of...
. Applications include: forming and characterizing new elements, determining the age of materials, and creating radioactive reagents for specific tracer use in tissues and organs. The ongoing goal of radioanalytical researchers is to develop more radionuclides and lower concentrations in people and the environment.
Alpha-Particle Decay
Alpha decayAlpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
is characterized by the emission of an alpha particle, a 4He nucleus. The mode of this decay causes the parent nucleus to decrease by two protons and two neutrons. This type of decay follows the relation:
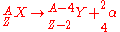
Beta-Particle Decay
Beta decayBeta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
is characterized by the emission of a neutrino
Neutrino
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
and a negatron which is equivalent to an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
. This process occurs when a nucleus has an excess of neutrons with respect to protons, as compared to the stable isobar
Isobar (nuclide)
Isobars are atoms of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number but not in mass number. An example of a series of isobars would be 40S, 40Cl, 40Ar, 40K, and 40Ca...
. This type of transition converts a neutron into a proton; similarly, a positron
Positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron...
is released when a proton is converted into a neutron. These decays follows the relation:
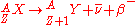
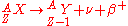
Gamma-Ray Decay
Gamma rayGamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
emission is follows the previously discussed modes of decay when the decay leaves a daughter nucleus in an excited state. This nucleus is capable of further de-excitation to a lower energy state by the release of a photon. This decay follows the relation:
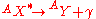
Gas Ionization Detectors
Gaseous ionization detectorsGaseous ionization detectors
In particle physics, gaseous ionization detectors are detectors designed to seek the presence of particles . If a particle has enough energy to ionize a gas atom or molecule, the resulting electrons and ions cause a current flow which can be measured in different ways...
collect and record the electrons freed from gaseous atoms and molecules by the interaction of radiation released by the source. A voltage potential
Potential
*In linguistics, the potential mood*The mathematical study of potentials is known as potential theory; it is the study of harmonic functions on manifolds...
is applied between two electrodes within a sealed system. Since the gaseous atoms are ionized after they interact with radiation they are attracted to the anode which produces a signal. It is important to vary the applied voltage such that the response falls within a critical proportional range.
Solid-State Detectors
The operating principle of Semiconductor detectorSemiconductor detector
This article is about particle detectors. For information about semiconductor detectors in radio, see Diode#Semiconductor_diodes, rectifier, detector and cat's-whisker detector....
s is similar to gas ionization detectors expect instead of ionization gas atoms, free electrons and holes are produced which create a signal at the electrodes. The advantage of solid state detectors is the greater resolution of the resultant energy spectrum. Usually NaI(Tl) detectors are used; for more precise applications Ge(Li) and Si(Li) detectors have been developed. For extra sensitive measurements high-pure germanium detectors are used under a liquid nitrogen environment.
Scintillation Detectors
ScintillationScintillation (physics)
Scintillation is a flash of light produced in a transparent material by an ionization event. See scintillator and scintillation counter for practical applications.-Overview:...
detectors uses a photo luminescent source (such as ZnS) which interacts with radiation. When a radioactive particle decays and strikes the photo luminescent material a photon is released. This photon is multiplied in a photomultiplier tube which converts light into an electrical signal. This signal is then processed and converted into a channel. By comparing the number of counts to the energy level (typically in keV or MeV) the type of decay can be determined.
Chemical Separation Techniques
Due to radioactive nucleotides have similar properties to their stable, inactive, counterparts similar analytical chemistryAnalytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
separation techniques can be used. These separation methods include precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
, Ion Exchange
Ion exchange
Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion...
, Liquid Liquid
Liquid Liquid
Liquid Liquid is a New York City post-punk, post-disco band, originally active from 1980 to 1983. They are perhaps best known for their track, "Cavern", which was covered by the Sugar Hill Records house band as the backing track for Grandmaster + Melle Mel's old school rap classic, "White Lines "...
extraction, Solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
Phase extraction, Distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
, and Electrodeposition
Electrodeposition
Electrodeposition may refer to:*Electroplating*Electrophoretic deposition*Underpotential deposition...
.
Sample Loss by Radiocolloidal Behaviour
Samples with very low concentrations are difficult to measure accurately due to the radioactive atoms unexpectedly depositing on surfaces. Sample loss at trace levels may be due to adhesion to container walls and filter surface sites by ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
ic or electrostatic adsorption, as well as metal foils and glass slides. Sample loss is an ever present concern, especially at the beginning of the analysis path where sequential steps may compound these losses.
Various solutions are known to circumvent these losses which include adding an inactive carrier
Carrier
Carrier may refer to:- Science :* Carrier wave, a waveform suitable for modulation by an information-bearing signal* Charge carrier, an unbound particle carrying an electric charge* a mathematical Set over which an algebraic structure is defined...
or adding a tracer
Tracer
Tracer may refer to:* Histochemical tracer, a substance used for tracing purposes in histochemistry, the study of the composition of cells and tissues...
. Research has also shown that that pretreatment of glassware and plastic surfaces can reduce radionuclide sorption by saturating the sites.
Carrier or Tracer Addition
Due to the inherent nature of radionuclides yielding low concentrations a common technique to improve yields is the addition of carrier ions or tracers. Isotope dilution involves the addition of a known amount of radionuclide tracer to the sample that contains a known stable element. This is done at the start of the analysis procedure so once the final measurements are taken, sample loss is considered. This procedure avoids the need for any quantitative recovery which greatly simplifies the analytical process.Carrier addition is the reverse technique of tracer addition. Instead of isotope dilution, a known mass of stable carrier ion is added to radionuclide sample solution. The carrier reagent must be calibrated prior to addition to the sample. To verify the resultant measurements, the expected 100% yield is compared to the actual yield. Any loss in yield is analogous to any losses in the radioactive sample. Typically the amount of carrier added is conventionally selected for the ease of weighing such that the accuracy of the resultant weight is within 1%. For alpha particles, special techniques must be applied to obtain the required thin sample sources.
Typical Radionuclides of Interest
| element Chemical element A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements... | mass Atomic mass The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom.... | half-life Half-life Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to... (years) | typical source |
|---|---|---|---|
| helium Helium Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table... |
3 | - stable - | air, water, and biota Biota (ecology) Biota are the total collection of organisms of a geographic region or a time period, from local geographic scales and instantaneous temporal scales all the way up to whole-planet and whole-timescale spatiotemporal scales. The biota of the Earth lives in the biosphere.-See... samples for bioassays |
| carbon Carbon Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds... |
14 | 5,730 | dating Radiocarbon dating Radiocarbon dating is a radiometric dating method that uses the naturally occurring radioisotope carbon-14 to estimate the age of carbon-bearing materials up to about 58,000 to 62,000 years. Raw, i.e. uncalibrated, radiocarbon ages are usually reported in radiocarbon years "Before Present" ,... of organic matter, water |
| iron Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
55 | 2.7 | produced in iron and steel casings, vessels, or supports for nuclear weapons and reactors |
| Strontium Strontium Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and... |
90 | 28.8 | common fission product |
| Technetium Technetium Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature... |
99 | 214,000 | another common fission product |
| iodine Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... |
129 | 15.7 million | groundwater tracer |
| Cesium | 137 | 30.2 | nuclear weapons and nuclear reactors (accidents) |
| Promethium Promethium Promethium is a chemical element with the symbol Pm and atomic number 61. It is notable for being the only exclusively radioactive element besides technetium that is followed by chemical elements with stable isotopes.- Prediction :... |
147 | 2.62 | naturally occurring fission product |
| Radon Radon Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days... |
226 | 1,600 | rain and groundwater, atmosphere |
| Uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
232, 233, 234, 235, 236, 238 | Varies | terrestrial element |
| Plutonium Plutonium Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation... |
238, 239, 240, 241, 242 | Varies | nuclear weapons and reactors |
| Americium Americium Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944... |
241 | 433 | result of neutron interactions with uranium and plutonium |
Quality Assurance
As this is an analytical chemistryAnalytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
technique quality control
Quality control
Quality control, or QC for short, is a process by which entities review the quality of all factors involved in production. This approach places an emphasis on three aspects:...
is an important factor to maintain. A laboratory
Laboratory
A laboratory is a facility that provides controlled conditions in which scientific research, experiments, and measurement may be performed. The title of laboratory is also used for certain other facilities where the processes or equipment used are similar to those in scientific laboratories...
must produce trustworthy results. This can be accomplished by a laboratories continual effort to maintain instrument
Scientific instrument
A scientific instrument can be any type of equipment, machine, apparatus or device as is specifically designed, constructed and often, through trial and error, ingeniously refined to apply utmost efficiency in the utilization of well proven physical principle, relationship or technology to...
calibration
Calibration
Calibration is a comparison between measurements – one of known magnitude or correctness made or set with one device and another measurement made in as similar a way as possible with a second device....
, measurement reproducibility, and applicability of analytical methods. In all laboratories there must be a quality assurance plan. This plan describes the quality system and procedures in place to obtain consistent results. Such results must be authentic, appropriately documented, and technically defensible." Such elements of quality assurance include organization, personnel training, laboratory operating procedures, procurement documents, chain of custody records, standard certificates, analytical records, standard procedures, QC sample analysis program and results, instrument testing and maintenance records, results of performance demonstration projects, results of data assessment, audit reports, and record retention policies.
The cost of quality assurance is continually on the rise but the benefits far outweigh this cost. The average quality assurance workload was risen from 10% to a modern load of 20-30%. This heightened focus on quality assurance ensures that quality measurements that are reliable are achieved. The cost of failure far outweighs the cost of prevention and appraisal. Finally, results must be scientifically defensible by adhering to stringent regulations in the event of a lawsuit.
Further reading
- Chemical Analysis by Nuclear Methods, by Z.B. Alfassi
- Radioanalytical chemistry by J. Tölgyessy, & M. Kyrš.
- Nuclear analytical chemistry by J. Tölgyessy, Š. Varga and V. Kriváň. English translation: P. Tkáč.

