
Polytropic process
Encyclopedia
A polytropic process is a thermodynamic process that obeys the relation:

where p is the pressure, V is volume, n, the polytropic index, is any real number
, and C is a constant. This equation can be used to accurately characterize processes of certain system
s, notably the compression
or expansion
of a gas
and in some cases liquid
s and solid
s.
and the values of the heat capacities, and
and 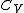 , are almost constant when
, are almost constant when  is not zero or infinity. (In reality,
is not zero or infinity. (In reality, 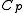 and
and 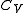 are actually functions of temperature and pressure, but are nearly constant within small changes of temperature).
are actually functions of temperature and pressure, but are nearly constant within small changes of temperature).
Under standard conditions, most gases can be accurately characterized by the ideal gas law
. This construct allows for the pressure-volume relationship to be defined for essentially all ideal thermodynamic cycles, such as the well-known Carnot cycle
. Note however that there may also be instances where a polytropic process occurs in a non-ideal gas.
When the index n is between any two of the former values (0, 1, gamma, or infinity), it means that the polytropic curve will bounded by
the curves of the two corresponding indices.
Note that , since
, since 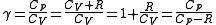 .
.
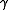 is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
An isothermal ideal gas is also a polytropic gas. Here, the polytropic index is equal to one, and differs from the adiabatic index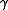 .
.
In order to discriminate between the two gammas, the polytropic gamma is sometimes capitalized,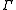 .
.
To confuse matters further, some authors refer to as the polytropic index, rather than
as the polytropic index, rather than  . Note that
. Note that

using a polytropic fluid is known as a polytrope.

where p is the pressure, V is volume, n, the polytropic index, is any real number
Real number
In mathematics, a real number is a value that represents a quantity along a continuum, such as -5 , 4/3 , 8.6 , √2 and π...
, and C is a constant. This equation can be used to accurately characterize processes of certain system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
s, notably the compression
Physical compression
Physical compression is the result of the subjection of a material to compressive stress, which results in reduction of volume as compared to an uncompressed but otherwise identical state. The opposite of compression in a solid is tension. In any medium transmitting waves, the opposite of...
or expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
of a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
and in some cases liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s and solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s.
Applicability
The equation is a valid characterization of a thermodynamic process assuming that the process is quasistaticQuasistatic equilibrium
Quasistatic equilibrium is the quasi-balanced state of a thermodynamic system near to thermodynamic equilibrium in some sense or degree...
and the values of the heat capacities,
 and
and 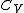 , are almost constant when
, are almost constant when  is not zero or infinity. (In reality,
is not zero or infinity. (In reality, 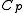 and
and 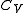 are actually functions of temperature and pressure, but are nearly constant within small changes of temperature).
are actually functions of temperature and pressure, but are nearly constant within small changes of temperature).Under standard conditions, most gases can be accurately characterized by the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
. This construct allows for the pressure-volume relationship to be defined for essentially all ideal thermodynamic cycles, such as the well-known Carnot cycle
Carnot cycle
The Carnot cycle is a theoretical thermodynamic cycle proposed by Nicolas Léonard Sadi Carnot in 1824 and expanded by Benoit Paul Émile Clapeyron in the 1830s and 40s. It can be shown that it is the most efficient cycle for converting a given amount of thermal energy into work, or conversely,...
. Note however that there may also be instances where a polytropic process occurs in a non-ideal gas.
Relationship to ideal processes
For certain values of the polytropic index, the process will be synonymous with other common processes. Some examples of the effects of varying index values are given in the table.| Polytropic index |
Relation | Effects |
|---|---|---|
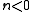 |
An explosion Explosion An explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. An explosion creates a shock wave. If the shock wave is a supersonic detonation, then the source of the blast is called a "high explosive"... occurs |
|
 |
 (constant) |
Equivalent to an isobaric process Isobaric process An isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,... (constant pressure Pressure Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :... ) |
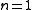 |
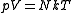 (constant) |
Equivalent to an isothermal process Isothermal process An isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir... (constant temperature Thermodynamic temperature Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the... ) |
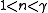 |
A quasi-adiabatic process such as in an internal combustion engine Internal combustion engine The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine... during expansion, or in vapor compression refrigeration during compression |
|
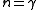 |
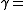 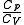 is the adiabatic index, yielding an adiabatic process is the adiabatic index, yielding an adiabatic processAdiabatic process In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,... (no heat Heat In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between... transferred) |
|
 |
Equivalent to an isochoric process Isochoric process An isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant... (constant volume) |
When the index n is between any two of the former values (0, 1, gamma, or infinity), it means that the polytropic curve will bounded by
Bounded function
In mathematics, a function f defined on some set X with real or complex values is called bounded, if the set of its values is bounded. In other words, there exists a real number M...
the curves of the two corresponding indices.
Note that
 , since
, since 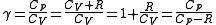 .
.Notation
In the case of an isentropic ideal gas,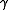 is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.An isothermal ideal gas is also a polytropic gas. Here, the polytropic index is equal to one, and differs from the adiabatic index
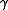 .
.In order to discriminate between the two gammas, the polytropic gamma is sometimes capitalized,
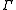 .
.To confuse matters further, some authors refer to
 as the polytropic index, rather than
as the polytropic index, rather than  . Note that
. Note that
Other
A solution to the Lane-Emden equationLane-Emden equation
In astrophysics, the Lane–Emden equation is Poisson's equation for the gravitational potential of a self-gravitating, spherically symmetric polytropic fluid. It is named after the astrophysicists Jonathan Homer Lane and Robert Emden...
using a polytropic fluid is known as a polytrope.
See also
- Adiabatic processAdiabatic processIn thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
- Isentropic processIsentropic processIn thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
- Isobaric processIsobaric processAn isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
- Isochoric processIsochoric processAn isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant...
- Isothermal processIsothermal processAn isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
- vapor compression refrigeration
- gas compressorGas compressorA gas compressor is a mechanical device that increases the pressure of a gas by reducing its volume.Compressors are similar to pumps: both increase the pressure on a fluid and both can transport the fluid through a pipe. As gases are compressible, the compressor also reduces the volume of a gas...
- internal combustion engineInternal combustion engineThe internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
- Quasistatic equilibriumQuasistatic equilibriumQuasistatic equilibrium is the quasi-balanced state of a thermodynamic system near to thermodynamic equilibrium in some sense or degree...
- ThermodynamicsThermodynamicsThermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...


