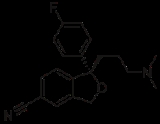
Skeletal formula
Overview
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
is a shorthand representation of its molecular structure
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
, developed by the organic chemist, Friedrich August Kekulé von Stradonitz
Friedrich August Kekulé von Stradonitz
Friedrich August Kekule von Stradonitz was a German organic chemist. From the 1850s until his death, Kekule was one of the most prominent chemists in Europe, especially in theoretical chemistry...
. Skeletal formulae are ubiquitous in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, because they are relatively quick and simple to draw. Carbon and hydrogen atoms are not shown explicitly. A skeletal formula shows the skeletal structure or skeleton of a molecule, which is composed of skeletal atoms.
The skeletal structure of an organic compound is the string of connected atoms that form the essential structure of the compound.
Unanswered Questions

