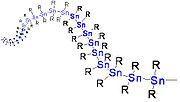
Polystannane
Encyclopedia

History
Oligo- or polystannanes were first described by Löwig in 1852, only 2 years after Edward FranklandEdward Frankland
Sir Edward Frankland, KCB, FRS was a chemist, one of the foremost of his day. He was an expert in water quality and analysis, and originated the concept of combining power, or valence, in chemistry. He was also one of the originators of organometallic chemistry.-Biography:Edward Frankland was born...
's report on the isolation of the first organotin
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849...
compounds. Löwig' route involved treating an Sn/K and Sn/Na alloys with iodoethane, in the presence of quartz sand which was used to control the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
. Products with elemental compositions close to those of oligo(diethylstannane)s or poly(diethylstannane) were obtained. Cahours obtained similar products and attributed the formation of the so-called "stannic ethyl" to a reaction of the Wurtz type
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
. Already in 1858, "stannic ethyl" was formulated as a polymeric compound denoted with the composition n(SnC4H5). In 1917 Grüttner, who reinvestigated results on hexaethyl-distannanes(H5C2)3Sn-Sn(C2H5)3 (reported by Ladenburg in 1870) confirmed the presence of Sn-Sn bonds and predicated for the first time that tin could form chain like compounds. In 1943, it was postulated that “diphenyltin” exists as a type of polymeric material because of its yellow color [8], and indeed a bathochromic shift
Bathochromic shift
Bathochromic shift is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength ....
of the wavelength at maximum absorption with increasing number of Sn atoms was found later in the case of oligo(dibutylstannane)s comprising up to 15 Sn atoms.
The Wurtz reaction
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
is still used for the preparation of poly(dialkylstannane)s. Treatment of dialkyltin dichlorides with sodium lead to polystannanes of high molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
, however, in low yields and with formation of (cyclic) oligomers. [11-16]. Other efforts to prepare high molar mass polystannanes by electrochemical reactions or by catalytic dehydropolymerization of dialkylstannanes (R2SnH2) were also made [16]. Unfortunately, frequently, the polymers prepared by those methods were not isolated and typically contained significant fractions of cyclic oligomers.
Linear polystannanes
Dialkytin dihydrides (R2SnH2) were reported in 2005 to undergo dehydropolymerization in the presence of Wilkinson’s catalyst
Wilkinson's catalyst
Wilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
. This method afforded polystannanes without detectable amounts of "cyclic"-byproducts. The polymers were yellow with number average molar masses of 10 to 70 kg/mol and a polydispersity of 2 – 3. By variation of the catalyst concentration the molar masses of the synthesized polymers could be adjusted. A strong influence of the temperature on the degree of conversion was observed. Determination of the molar mass at different degrees of conversion indicated that polymerization did not proceed according to a statistical condensation mechanism, but, likely, by growth onto the catalyst, e.g. by insertion of SnR2-like units.
The poly(dialkylstannane)s were found to be thermotropic
Thermotropic
A liquid crystal is thermotropic if the order of its components is determined or changed by temperature.If temperature is too high, the rise in energy and therefore in motion of the components will induce a phase change: the LC will become an isotropic liquid.If, on the contrary, temperature is...
and displayed first-order phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
s from one liquid-crystalline
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
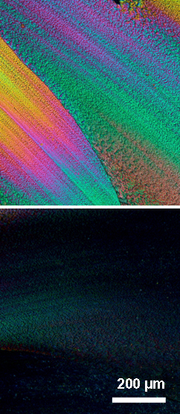
phase into another or directly to the isotropic state, depending on the length of the side groups. More specifically, poly(dibutylstannane) for example showed an endothermic phase transition at ~0 °C from a rectangular to a pure nematic phase, as determined by X-ray diffraction.
As expected, polystannanes were semi-conductive. Temperature-dependent, time-resolved pulse radiolysis
Radiolysis
Radiolysis is the dissociation of molecules by nuclear radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux...
microwave conductivity measurements of poly(dibutylstannane) yielded values of charge-carrier mobilities of 0.1 to 0.03 cm2 V−1 s−1, which are similar to those found for pi-bond-conjugated carbon-based polymers. By partial oxidation of the material with SbF5 conductivities of 0.3 S cm−1 could be monitored.
The liquid-crystalline characteristics of the poly(dialkylstannane)s permitted facile orientation of these macromolecules, for instance, by mechanical shearing or tensile drawing of blends with poly(ethylene). Poly(dialkylstannane)s with short side groups invariably arranged parallel to the external orientation direction, while the polymers with longer side groups had a tendency to order themselves perpendicular to that axis.
External links
- Fabien Choffat (2007) Polystannane, Doctoral dissertation, Swiss Federal Institute of Technology, Zürich.