Molecular solid
Encyclopedia
Molecular solid is a solid
composed of molecules held together by the van der Waals force
s. Because these dipole
forces are weaker than covalent or ionic bonds
, molecular solids are soft and have relatively low melting temperature
. Pure molecular solids are electrical insulators but they can be made conductive by doping
. Examples of molecular solids include hydrocarbon
s, ice
, sugar
, fullerene
s, sulfur
and solid carbon dioxide
.
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
composed of molecules held together by the van der Waals force
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
s. Because these dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
forces are weaker than covalent or ionic bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
, molecular solids are soft and have relatively low melting temperature
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
. Pure molecular solids are electrical insulators but they can be made conductive by doping
Doping (semiconductor)
In semiconductor production, doping intentionally introduces impurities into an extremely pure semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic...
. Examples of molecular solids include hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s, ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
, sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
, fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s, sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and solid carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
.
Structure and composition
higher alkanesHigher alkanes
Higher alkanes are often defined as alkanes having nine or more carbon atoms. Nonane is the lightest alkane to have a flash point above 25 °C, and so not to be classified as dangerously flammable....
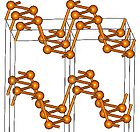
The term "molecular solid" may refer not to a certain chemical composition, but to a specific form of a material. For example, solid phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
can crystallize in different allotropes called "white", "red" and "black" phosphorus. White phosphorus forms molecular crystals composed of tetrahedral P4 molecules. Heating at ambient pressure to 250 °C or exposing to sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
converts white phosphorus to red phosphorus where the P4 tetrahedra are no longer isolated, but are connected by covalent bonds into polymer-like chains. Heating white phosphorus under high (GPa) pressures converts it to black phosphorus which has a layered, graphite-like structure.
The structural transitions in phosphorus are reversible: upon releasing high pressure, black phosphorus gradually converts into the red allotrope, and by vaporizing red phosphorus at 490 °C in inert atmosphere and condensing the vapor, covalent red phosphorus can be transformed back into the white molecular solid.
 |
 |
 |
|
| White, red, violet and black phosphorus samples | Structure unit of white phosphorus |
Structures of red | and black phosphorus |
Similarly, yellow arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
is a molecular solid composed of As4 units; it is metastable and gradually transforms into gray arsenic upon heating or illumination. Some forms of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
are composed of S8 (or Se8) units and are molecular solids at ambient conditions, but they can convert into covalent allotropes having atomic chains extending all through the crystal.
Changes in the chemical composition can have even stronger effects on the bonding in solids. For example, whereas both hydrogen and lithium belong to the first group of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, LiCl
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
is ionic and HCl
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
is a molecular solid.
Examples of molecular solids
Several classes of molecular solids can be distinguished (see table right). The vast majority of molecular solids can be attributed to organic compounds containing carbon and hydrogen, such as hydrocarbons (CnHm). Spherical molecules consisting of different number of carbon atoms, that is fullereneFullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s, are another important class. Less numerous, yet distinctive molecular solids are halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s (e.g. Cl2) and their compounds with hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(HCl), as well as light chalcogen
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
s (O2) and pnictogens
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
(N2).
Properties
Weakness of intermolecular forces results in low melting temperatures of molecular solids. Whereas the characteristic melting pointMelting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
of metals and ionic solids is ~1000 °C, most molecular solids melt well below ~300 °C (see table), thus many corresponding substances are either liquid (ice) or gaseous (oxygen) at room temperature. Molecular solids also have relatively low density and hardness. This is because of the light elements involved and relatively long and thus weak intermolecular bonds. Because of the charge neutrality of the constituent molecules and long distance between them, molecular solids are electrical insulators.
The above tendencies can be illustrated on example of different allotropes of phosphorus. White phosphorus, a molecular solid, has a relatively low density of 1.82 g/cm3 and melting point of 44.1 °C; it is a soft material which can be cut with a knife. When it is converted to the covalent red phosphorus, the density increases to 2.2–2.4 g/cm3 and melting point to 590 °C, and when white phosphorus is transformed into the (also covalent) black phosphorus, the density becomes 2.69–3.8 g/cm3 and melting temperature ~200 °C. Both red and black phosphorus forms are significantly harder than white phosphorus, and whereas white phosphorus is an insulator, the black allotrope, which consists of layers extending over the whole crystal, does conduct electricity.
Conductivity of molecular solids can be illustrated on example of fullerene. Its solid is an insulator because all valence electrons of carbon atoms are involved into the covalent bonds within the individual carbon molecules. However, inserting (intercalating) alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
atoms between the fullerene molecules provides extra electrons, which can be easily ionized from the metal atoms and make material conductive and even superconductive
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
.

