Mineral redox buffer
Encyclopedia
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity
as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.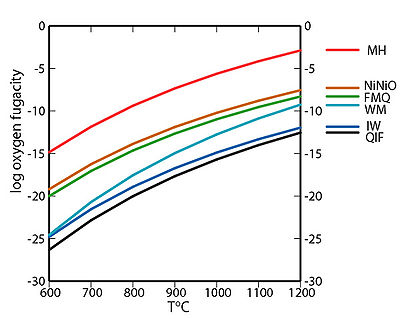 For other rocks with suitable minerals, oxygen fugacities can be calculated, and the redox conditions can be obtained by comparison to the fugacity-temperature diagram.
For other rocks with suitable minerals, oxygen fugacities can be calculated, and the redox conditions can be obtained by comparison to the fugacity-temperature diagram.
Redox buffers were developed in part to control oxygen fugacities in laboratory experiments to investigate mineral stabilities and rock histories. Each of the curves plotted in the fugacity-temperature diagram is for an oxidation reaction occurring in a buffer. These redox buffers are listed here in order of decreasing oxygen fugacity at a given temperature—in other words, from more oxidizing to more reducing conditions in the plotted temperature range. As long as all the pure minerals (or compounds) are present in a buffer assemblage, the oxidizing conditions are fixed on the curve for that buffer. Pressure has only a minor influence on these buffer curves for conditions in the Earth's crust
.
MH magnetite
-hematite
4 Fe3O4 + O2 = 6 Fe2O3
NiNiO nickel
-nickel oxide
2 Ni + O2 = 2 NiO
FMQ fayalite
-magnetite
-quartz
3 Fe2SiO4 +O2 = 2 Fe3O4 + 3 SiO2
WM wustite
-magnetite
3 Fe1-xO + O2 ~ Fe3O4
IW iron
-wustite
2(1-x) Fe + O2 = 2 Fe1-xO
QIF quartz
-iron
-fayalite
2 Fe + SiO2 + O2 = Fe2SiO4
and oxide mineral
assemblage of the rock. Within a rock of a given chemical composition, iron enters minerals based on the bulk chemical composition and the mineral phases which are stable at that temperature and pressure. For instance, at redox conditions more oxidizing than the MH (magnetite-hematite) buffer, at least much of the iron is likely to be present as Fe3+ and hematite
is a likely mineral in iron-bearing rocks. Iron may only enter minerals such as olivine
if it is present as Fe2+; Fe3+ cannot enter the lattice of fayalite
olivine. Elements in olivine such as magnesium
, however, stabilize olivine containing Fe2+ to conditions more oxidizing than those required for fayalite stability. Solid solution
between magnetite and the titanium
-bearing endmember
, ulvospinel
, enlarges the stability field of magnetite. Likewise, at conditions more reducing than the IW (iron-wustite) buffer, minerals such as pyroxene can still contain Fe3+. The redox buffers therefore are only approximate guides to the proportions of Fe2+ and Fe3+ in minerals and rocks.
s commonly record crystallization at oxygen fugacities
more oxidizing than the WM (wüstite
-magnetite
) buffer and more reduced than a log unit or so above the nickel-nickel oxide (NiNiO) buffer. Their oxidizing conditions thus are not far from those of the FMQ (fayalite
-magnetite
-quartz
) redox buffer. Nonetheless, there are systematic differences that correlate with tectonic setting. Igneous rock
emplaced and erupted in island arc
s typically record oxygen fugacities 1 or more log units more oxidizing than those of the NiNiO buffer. In contrast, basalt
and gabbro
in non-arc settings typically record oxygen fugacities from about those of the FMQ buffer to a log unit or so more reducing than that buffer.
and diagenesis of sedimentary rocks. The fugacity of oxygen at the MH buffer (magnetite
-hematite
) is only about 10-70 at 25°C, but it is about 0.2 atmospheres in the Earth's atmosphere
, so some sedimentary environments are far more oxidizing than those in magmas. Other sedimentary environments, such as the environments for formation of black shale
, are relatively reducing.
extend to higher values than those in magmatic environments, because of the more oxidizing compositions inherited from some sedimentary rocks. Nearly pure hematite is present in some metamorphosed banded iron formation
s. In contrast, native nickel-iron is present in some serpentinite
s.
s, the iron
-wüstite
redox buffer may be more appropriate for describing the oxygen fugacity of these extraterrestrial systems.
minerals such as pyrite
(FeS2) and pyrrhotite
(Fe1-xS) occur in many ore deposits. Pyrite and its polymorph
marcasite
also are important in many coal
deposits and shales
. These sulfide minerals form in environments more reducing than that of the Earth's surface. When in contact with oxidizing surface waters, sulfides react: sulfate (SO4--) forms, and the water becomes acidic and charged with a variety of elements, some potentially toxic. Consequences can be environmentally harmful, as discussed in the entry for acid mine drainage
.
Sulfur oxidation to sulfate also is important in generating sulfur-rich volcanic eruptions, like those of Pinatubo in 1991 and El Chichon
in 1982. These eruptions contributed unusually large quantities of sulfur to the Earth's atmosphere
, with consequent effects on atmospheric quality and on climate. The magma
s were unusually oxidizing, almost two log units more so than the NiNiO buffer. The calcium sulfate
, anhydrite
, was present as phenocryst
s in the erupted tephra
. In contrast, sulfides contain most of the sulfur in magmas more reducing than the FMQ buffer.
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

Common redox buffers and mineralogy
Common redox buffersRedox buffers were developed in part to control oxygen fugacities in laboratory experiments to investigate mineral stabilities and rock histories. Each of the curves plotted in the fugacity-temperature diagram is for an oxidation reaction occurring in a buffer. These redox buffers are listed here in order of decreasing oxygen fugacity at a given temperature—in other words, from more oxidizing to more reducing conditions in the plotted temperature range. As long as all the pure minerals (or compounds) are present in a buffer assemblage, the oxidizing conditions are fixed on the curve for that buffer. Pressure has only a minor influence on these buffer curves for conditions in the Earth's crust
Crust (geology)
In geology, the crust is the outermost solid shell of a rocky planet or natural satellite, which is chemically distinct from the underlying mantle...
.
MH magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
-hematite
Hematite
Hematite, also spelled as haematite, is the mineral form of iron oxide , one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum...
4 Fe3O4 + O2 = 6 Fe2O3
NiNiO nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
-nickel oxide
2 Ni + O2 = 2 NiO
FMQ fayalite
Fayalite
Fayalite is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system with cell parameters a 4.82 Å, b 10.48 Å and c Å 6.09.Iron rich olivine is a relatively common constituent of acidic and...
-magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
-quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
3 Fe2SiO4 +O2 = 2 Fe3O4 + 3 SiO2
WM wustite
Wüstite
Wüstite is a mineral form of iron oxide found with meteorites and native iron. It has a gray color with a greenish tint in reflected light. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a...
-magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
3 Fe1-xO + O2 ~ Fe3O4
IW iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-wustite
Wüstite
Wüstite is a mineral form of iron oxide found with meteorites and native iron. It has a gray color with a greenish tint in reflected light. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a...
2(1-x) Fe + O2 = 2 Fe1-xO
QIF quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
-iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-fayalite
Fayalite
Fayalite is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system with cell parameters a 4.82 Å, b 10.48 Å and c Å 6.09.Iron rich olivine is a relatively common constituent of acidic and...
2 Fe + SiO2 + O2 = Fe2SiO4
Mineralogy and correlations with redox buffer
The ratio of Fe2+ to Fe3+ within a rock determines, in part, the silicate mineralSilicate minerals
The silicate minerals make up the largest and most important class of rock-forming minerals, constituting approximately 90 percent of the crust of the Earth. They are classified based on the structure of their silicate group...
and oxide mineral
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
assemblage of the rock. Within a rock of a given chemical composition, iron enters minerals based on the bulk chemical composition and the mineral phases which are stable at that temperature and pressure. For instance, at redox conditions more oxidizing than the MH (magnetite-hematite) buffer, at least much of the iron is likely to be present as Fe3+ and hematite
Hematite
Hematite, also spelled as haematite, is the mineral form of iron oxide , one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum...
is a likely mineral in iron-bearing rocks. Iron may only enter minerals such as olivine
Olivine
The mineral olivine is a magnesium iron silicate with the formula 2SiO4. It is a common mineral in the Earth's subsurface but weathers quickly on the surface....
if it is present as Fe2+; Fe3+ cannot enter the lattice of fayalite
Fayalite
Fayalite is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system with cell parameters a 4.82 Å, b 10.48 Å and c Å 6.09.Iron rich olivine is a relatively common constituent of acidic and...
olivine. Elements in olivine such as magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, however, stabilize olivine containing Fe2+ to conditions more oxidizing than those required for fayalite stability. Solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
between magnetite and the titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
-bearing endmember
Endmember (mineralogy)
An endmember in mineralogy is a mineral that is at the extreme end of a mineral series in terms of purity. Minerals often can be described as solid solutions with varying compositions of some chemical elements, rather than as substances with an exact chemical formula...
, ulvospinel
Ulvöspinel
Ulvöspinel or ulvite is an iron titanium oxide mineral with formula: Fe2TiO4 or TiFe2+2O4. It forms brown to black metallic isometric crystals with a Mohs hardness of 5.5 to 6...
, enlarges the stability field of magnetite. Likewise, at conditions more reducing than the IW (iron-wustite) buffer, minerals such as pyroxene can still contain Fe3+. The redox buffers therefore are only approximate guides to the proportions of Fe2+ and Fe3+ in minerals and rocks.
Igneous Rocks
Terrestrial igneous rockIgneous rock
Igneous rock is one of the three main rock types, the others being sedimentary and metamorphic rock. Igneous rock is formed through the cooling and solidification of magma or lava...
s commonly record crystallization at oxygen fugacities
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
more oxidizing than the WM (wüstite
Wüstite
Wüstite is a mineral form of iron oxide found with meteorites and native iron. It has a gray color with a greenish tint in reflected light. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a...
-magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
) buffer and more reduced than a log unit or so above the nickel-nickel oxide (NiNiO) buffer. Their oxidizing conditions thus are not far from those of the FMQ (fayalite
Fayalite
Fayalite is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system with cell parameters a 4.82 Å, b 10.48 Å and c Å 6.09.Iron rich olivine is a relatively common constituent of acidic and...
-magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
-quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
) redox buffer. Nonetheless, there are systematic differences that correlate with tectonic setting. Igneous rock
Igneous rock
Igneous rock is one of the three main rock types, the others being sedimentary and metamorphic rock. Igneous rock is formed through the cooling and solidification of magma or lava...
emplaced and erupted in island arc
Island arc
An island arc is a type of archipelago composed of a chain of volcanoes which alignment is arc-shaped, and which are situated parallel and close to a boundary between two converging tectonic plates....
s typically record oxygen fugacities 1 or more log units more oxidizing than those of the NiNiO buffer. In contrast, basalt
Basalt
Basalt is a common extrusive volcanic rock. It is usually grey to black and fine-grained due to rapid cooling of lava at the surface of a planet. It may be porphyritic containing larger crystals in a fine matrix, or vesicular, or frothy scoria. Unweathered basalt is black or grey...
and gabbro
Gabbro
Gabbro refers to a large group of dark, coarse-grained, intrusive mafic igneous rocks chemically equivalent to basalt. The rocks are plutonic, formed when molten magma is trapped beneath the Earth's surface and cools into a crystalline mass....
in non-arc settings typically record oxygen fugacities from about those of the FMQ buffer to a log unit or so more reducing than that buffer.
Sedimentary rocks
Oxidizing conditions are common in some environments of depositionSedimentary depositional environment
In geology, sedimentary depositional environment describes the combination of physical, chemical and biological processes associated with the deposition of a particular type of sediment and, therefore, the rock types that will be formed after lithification, if the sediment is preserved in the rock...
and diagenesis of sedimentary rocks. The fugacity of oxygen at the MH buffer (magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
-hematite
Hematite
Hematite, also spelled as haematite, is the mineral form of iron oxide , one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum...
) is only about 10-70 at 25°C, but it is about 0.2 atmospheres in the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
, so some sedimentary environments are far more oxidizing than those in magmas. Other sedimentary environments, such as the environments for formation of black shale
Shale
Shale is a fine-grained, clastic sedimentary rock composed of mud that is a mix of flakes of clay minerals and tiny fragments of other minerals, especially quartz and calcite. The ratio of clay to other minerals is variable. Shale is characterized by breaks along thin laminae or parallel layering...
, are relatively reducing.
Metamorphic rocks
Oxygen fugacities during metamorphismMetamorphism
Metamorphism is the solid-state recrystallization of pre-existing rocks due to changes in physical and chemical conditions, primarily heat, pressure, and the introduction of chemically active fluids. Mineralogical, chemical and crystallographic changes can occur during this process...
extend to higher values than those in magmatic environments, because of the more oxidizing compositions inherited from some sedimentary rocks. Nearly pure hematite is present in some metamorphosed banded iron formation
Banded iron formation
Banded iron formations are distinctive units of sedimentary rock that are almost always of Precambrian age. A typical BIF consists of repeated, thin layers of iron oxides, either magnetite or hematite , alternating with bands of iron-poor shale and chert...
s. In contrast, native nickel-iron is present in some serpentinite
Serpentinite
Serpentinite is a rock composed of one or more serpentine group minerals. Minerals in this group are formed by serpentinization, a hydration and metamorphic transformation of ultramafic rock from the Earth's mantle...
s.
Extraterrestrial rocks
Within meteoriteMeteorite
A meteorite is a natural object originating in outer space that survives impact with the Earth's surface. Meteorites can be big or small. Most meteorites derive from small astronomical objects called meteoroids, but they are also sometimes produced by impacts of asteroids...
s, the iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-wüstite
Wüstite
Wüstite is a mineral form of iron oxide found with meteorites and native iron. It has a gray color with a greenish tint in reflected light. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a...
redox buffer may be more appropriate for describing the oxygen fugacity of these extraterrestrial systems.
Redox effects and sulfur
SulfideSulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
minerals such as pyrite
Pyrite
The mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool's gold because of its resemblance to gold...
(FeS2) and pyrrhotite
Pyrrhotite
Pyrrhotite is an unusual iron sulfide mineral with a variable iron content: FeS . The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic...
(Fe1-xS) occur in many ore deposits. Pyrite and its polymorph
Polymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
marcasite
Marcasite
The mineral marcasite, sometimes called white iron pyrite, is iron sulfide with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures do have in common that they contain the disulfide...
also are important in many coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
deposits and shales
Shalës
Shalës is a municipality in the Elbasan District, Elbasan County, central Albania. The municipality consists of the villages Shalës, Licaj, Kurtalli, Xibrake, Xherie and Kodras....
. These sulfide minerals form in environments more reducing than that of the Earth's surface. When in contact with oxidizing surface waters, sulfides react: sulfate (SO4--) forms, and the water becomes acidic and charged with a variety of elements, some potentially toxic. Consequences can be environmentally harmful, as discussed in the entry for acid mine drainage
Acid mine drainage
Acid mine drainage , or acid rock drainage , refers to the outflow of acidic water from metal mines or coal mines. However, other areas where the earth has been disturbed may also contribute acid rock drainage to the environment...
.
Sulfur oxidation to sulfate also is important in generating sulfur-rich volcanic eruptions, like those of Pinatubo in 1991 and El Chichon
El Chichón
El Chichón, also known as El Chichonal is an active volcano in Francisco León Municipality in northwestern Chiapas, Mexico. Its only recorded eruptive activity was on March 29, April 3 and April 4, 1982 , when it produced a one km-wide caldera that then filled with an acidic crater lake...
in 1982. These eruptions contributed unusually large quantities of sulfur to the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
, with consequent effects on atmospheric quality and on climate. The magma
Magma
Magma is a mixture of molten rock, volatiles and solids that is found beneath the surface of the Earth, and is expected to exist on other terrestrial planets. Besides molten rock, magma may also contain suspended crystals and dissolved gas and sometimes also gas bubbles. Magma often collects in...
s were unusually oxidizing, almost two log units more so than the NiNiO buffer. The calcium sulfate
Calcium sulfate
Calcium sulfate is a common laboratory and industrial chemical. In the form of γ-anhydrite , it is used as a desiccant. It is also used as a coagulant in products like tofu. In the natural state, unrefined calcium sulfate is a translucent, crystalline white rock...
, anhydrite
Anhydrite
Anhydrite is a mineral – anhydrous calcium sulfate, CaSO4. It is in the orthorhombic crystal system, with three directions of perfect cleavage parallel to the three planes of symmetry. It is not isomorphous with the orthorhombic barium and strontium sulfates, as might be expected from the...
, was present as phenocryst
Phenocryst
thumb|right|300px|[[Granite]]s often have large [[feldspar|feldspatic]] phenocrysts. This granite, from the [[Switzerland|Swiss]] side of the [[Mont Blanc]] massif, has large white [[plagioclase]] phenocrysts, [[triclinic]] [[mineral]]s that give [[trapezium|trapezoid]] shapes when cut through)...
s in the erupted tephra
Tephra
200px|thumb|right|Tephra horizons in south-central [[Iceland]]. The thick and light coloured layer at center of the photo is [[rhyolitic]] tephra from [[Hekla]]....
. In contrast, sulfides contain most of the sulfur in magmas more reducing than the FMQ buffer.
See also
- WüstiteWüstiteWüstite is a mineral form of iron oxide found with meteorites and native iron. It has a gray color with a greenish tint in reflected light. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a...
, magnetiteMagnetiteMagnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
, hematiteHematiteHematite, also spelled as haematite, is the mineral form of iron oxide , one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum... - PyritePyriteThe mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool's gold because of its resemblance to gold...
, pyrrhotitePyrrhotitePyrrhotite is an unusual iron sulfide mineral with a variable iron content: FeS . The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic... - Normative mineralogyNormative mineralogyNormative mineralogy is a geochemical calculation of the whole rock geochemistry of a rock sample that estimates the idealised mineralogy of a rock according to the principles of geochemistry....
- Ellingham diagramEllingham diagramAn Ellingham diagram is a graph showing the temperature dependence of the stability for compounds. This analysis is usually used to evaluate the ease of reduction of metal oxides and sulphides. These diagrams were first constructed by Harold Ellingham in 1944...

