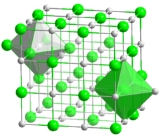
Wüstite
Encyclopedia
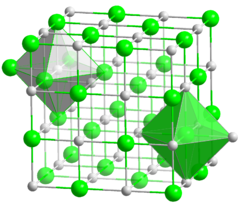
Iron(II) oxide
Iron oxide, also known as ferrous oxide, is one of the iron oxides. It is a black-colored powder with the chemical formula . It consists of the chemical element iron in the oxidation state of 2 bonded to oxygen. Its mineral form is known as wüstite. Iron oxide should not be confused with rust,...
found with meteorite
Meteorite
A meteorite is a natural object originating in outer space that survives impact with the Earth's surface. Meteorites can be big or small. Most meteorites derive from small astronomical objects called meteoroids, but they are also sometimes produced by impacts of asteroids...
s and native iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. It has a gray color with a greenish tint in reflected light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a specific gravity
Specific gravity
Specific gravity is the ratio of the density of a substance to the density of a reference substance. Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance. The reference substance is nearly always water for...
of 5.88. Wüstite is a typical example of a non-stoichiometric compound
Non-stoichiometric compound
Non-stoichiometric compounds are chemical compounds with an elemental composition that cannot be represented by a ratio of well-defined natural numbers, and therefore violate the law of definite proportions. Often, they are solids that contain crystallographic point defects, such as interstitial...
.
Wüstite was named for Fritz Wüst (1860–1938), a German metallurgist and founding director of the Kaiser-Wilhelm-Institut für Eisenforschung (presently Max Planck Institute for Iron Research GmbH
Max Planck Institute for Iron Research GmbH
The Max Planck Institute for Iron Research GmbH is a research institute of the Max Planck Society located in Düsseldorf. The institute was founded as Kaiser Wilhelm Institute for Iron Research in Aachen 1917 and moved 1921 to Düsseldorf.-External links:* *...
).
In addition to the type locality in Germany, it has been reported from Disko Island
Disko Island
Disko Island is a large island in Baffin Bay, off the west coast of Greenland. It has an area of , making it the second largest island of Greenland and one of the 100 largest islands in the world...
, Greenland; the Jharia
Jharia
Jharia is a notified area and one of eight development blocks in Dhanbad district in Jharkhand state, India. More than one town in India shares this name.- City :...
coalfield, Jharkhand
Jharkhand
Jharkhand is a state in eastern India. It was carved out of the southern part of Bihar on 15 November 2000. Jharkhand shares its border with the states of Bihar to the north, Uttar Pradesh and Chhattisgarh to the west, Orissa to the south, and West Bengal to the east...
, India and as inclusions in diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
s in a number of kimberlite
Kimberlite
Kimberlite is a type of potassic volcanic rock best known for sometimes containing diamonds. It is named after the town of Kimberley in South Africa, where the discovery of an diamond in 1871 spawned a diamond rush, eventually creating the Big Hole....
pipes. It also is reported from deep sea manganese nodule
Manganese nodule
Polymetallic nodules, also called manganese nodules, are rock concretions on the sea bottom formed of concentric layers of iron and manganese hydroxides around a core. The core may be microscopically small and is sometimes completely transformed into manganese minerals by crystallization...
s.
Its presence indicates a highly reducing
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
environment.
Wüstite Redox Buffer
- Main article: Mineral redox bufferMineral redox bufferIn geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions at which a rock forms and evolves can be important for interpreting the rock history...
Wüstite, in geochemistry, defines a redox buffer of oxidation within rocks at which point the rock is so reduced that Fe3+ and thus hematite
Hematite
Hematite, also spelled as haematite, is the mineral form of iron oxide , one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum...
is absent.
As the redox state of a rock is further reduced, magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
is converted to wüstite. This occurs by conversion of the Fe3+ ions in magnetite to Fe2+ ions. An example reaction is presented below:
- FeO.Fe2O3 + C --> 3FeO + CO
- magnetite + graphite/diamond --> wüstite + carbon monoxide
The formula for magnetite is more accurately written as FeO.Fe2O3 than as Fe3O4. Magnetite is one part FeO and one part Fe2O3, rather than a solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
of wüstite and hematite. The magnetite is termed a redox buffer because until all Fe3+ magnetite is converted to Fe2+ the oxide mineral assemblage of iron remains wüstite-magnetite, and furthermore the redox state of the rock remains at the same level of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
fugacity
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
. This is similar to buffering in the H+/OH- acid-base system of water.
Once the Fe3+ is consumed, then oxygen must be stripped from the system to further reduce it and wüstite is converted to native iron. The oxide mineral equilibrium assemblage of the rock becomes wüstite-magnetite-iron.
In nature, the only natural systems which are chemically reduced enough to even attain a wüstite-magnetite composition are rare, including carbonate-rich skarn
Skarn
Skarn is an old Swedish mining term originally used to describe a type of silicate gangue, or waste rock, associated with iron-ore bearing sulfide deposits apparently replacing Archean age limestones in Sweden's Persberg mining district. In modern usage the term "skarn" has been expanded to refer...
s, meteorites and perhaps the mantle where reduced carbon is present, exemplified by the presence of diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
and/or graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
.
Effects upon silicate minerals
The ratio of Fe2+ to Fe3+ within a rock determines, in part, the silicate mineral assemblage of the rock. Within a rock of a given chemical composition, iron enters minerals based on the bulk chemical composition and the mineral phases which are stable at that temperature and pressure. Iron may only enter minerals such as pyroxenePyroxene
The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. They share a common structure consisting of single chains of silica tetrahedra and they crystallize in the monoclinic and orthorhombic systems...
and olivine
Olivine
The mineral olivine is a magnesium iron silicate with the formula 2SiO4. It is a common mineral in the Earth's subsurface but weathers quickly on the surface....
if it is present as Fe2+; Fe3+ cannot enter the lattice of fayalite olivine and thus for every two Fe3+ ions, one Fe2+ is used and one molecule of magnetite is created.
In chemically reduced rocks, magnetite may be absent due to the propensity of iron to enter olivine, and wüstite may only be present if there is an excess of iron above what can be used by silica. Thus, wüstite may only be found in silica-undersaturated compositions which are also heavily chemically reduced, satisfying both the need to remove all Fe3+ and to maintain iron outside of silicate minerals.
In nature, carbonate rocks, potentially carbonatite
Carbonatite
Carbonatites are intrusive or extrusive igneous rocks defined by mineralogic composition consisting of greater than 50 percent carbonate minerals. Carbonatites may be confused with marble, and may require geochemical verification....
, kimberlite
Kimberlite
Kimberlite is a type of potassic volcanic rock best known for sometimes containing diamonds. It is named after the town of Kimberley in South Africa, where the discovery of an diamond in 1871 spawned a diamond rush, eventually creating the Big Hole....
s, carbonate-bearing melilitic rocks and other rare alkaline rocks may satisfy these criteria. However, wüstite is not reported in most of these rocks in nature, potentially because the redox state necessary to drive magnetite to wüstite is so rare.
Related minerals
Wüstite forms a solid solution with periclasePericlase
Periclase occurs naturally in contact metamorphic rocks and is a major component of most basic refractory bricks. It is a cubic form of magnesium oxide ....
(Mg
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
O), and Fe substitutes for Mg. Periclase, when hydrated, forms brucite
Brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Mg2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal vein mineral in metamorphosed limestones and chlorite schists; and formed during serpentinization of dunites...
(Mg(OH
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
)2), a common product of serpentinite
Serpentinite
Serpentinite is a rock composed of one or more serpentine group minerals. Minerals in this group are formed by serpentinization, a hydration and metamorphic transformation of ultramafic rock from the Earth's mantle...
metamorphic reaction
Metamorphic reaction
A metamorphic reaction is a chemical reaction that takes place during the geological process of metamorphism wherein one assemblage of minerals is transformed into a second assemblage which is stable under the new temperature/pressure conditions resulting in the final stable state of the observed...
s.
Oxidation of wüstite forms goethite-limonite.
Zinc, aluminium and other transition metals may substitute for Fe in wüstite.
Wüstite in dolomite
Dolomite
Dolomite is a carbonate mineral composed of calcium magnesium carbonate CaMg2. The term is also used to describe the sedimentary carbonate rock dolostone....
skarn
Skarn
Skarn is an old Swedish mining term originally used to describe a type of silicate gangue, or waste rock, associated with iron-ore bearing sulfide deposits apparently replacing Archean age limestones in Sweden's Persberg mining district. In modern usage the term "skarn" has been expanded to refer...
s may be related to siderite
Siderite
Siderite is a mineral composed of iron carbonate FeCO3. It takes its name from the Greek word σίδηρος sideros, “iron”. It is a valuable iron mineral, since it is 48% iron and contains no sulfur or phosphorus...
(Fe-carbonate), wollastonite
Wollastonite
Wollastonite is a calcium inosilicate mineral that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or dolostone is subjected to high temperature and pressure sometimes in the presence of silica-bearing fluids...
, enstatite
Enstatite
Enstatite is the magnesium endmember of the pyroxene silicate mineral series enstatite - ferrosilite . The magnesium rich members of the solid solution series are common rock-forming minerals found in igneous and metamorphic rocks...
, diopside
Diopside
Diopside is a monoclinic pyroxene mineral with composition MgCaSi2O6. It forms complete solid solution series with hedenbergite and augite, and partial solid solutions with orthopyroxene and pigeonite. It forms variably colored, but typically dull green crystals in the monoclinic prismatic class...
and magnesite
Magnesite
Magnesite is magnesium carbonate, MgCO3. Iron substitutes for magnesium with a complete solution series with siderite, FeCO3. Calcium, manganese, cobalt, and nickel may also occur in small amounts...
.

