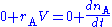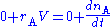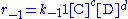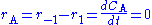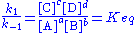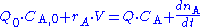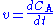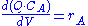
Mass balance
Encyclopedia
A mass balance is an application of conservation of mass
to the analysis of physical systems. By accounting for material entering and leaving a system, mass flow
s can be identified which might have been unknown, or difficult to measure without this technique. The exact conservation law
used in the analysis of the system depends on the context of the problem but all revolve around mass conservation, i.e. that matter
cannot disappear or be created spontaneously.
Therefore, mass balances are used widely in engineering
and environmental analyses. For example mass balance theory is used to design chemical reactor
s, analyse alternative processes to produce chemicals as well as in pollution
dispersion models and other models of physical systems. Closely related and complementary analysis techniques include the population balance
, energy balance
and the somewhat more complex entropy
balance. These techniques are required for thorough design and analysis of systems such as the refrigeration cycle.
In environmental monitoring the term budget calculations is used to describe mass balance equations where they are used to evaluate the monitoring data (comparing input and output, etc.) In biology the dynamic energy budget
theory for metabolic organisation makes explicit use of time, mass and energy balances.
Mathematically the mass balance for a system without a chemical reaction is as follows:
Strictly speaking the above equation holds also for systems with chemical reactions if the terms in the balance equation are taken to refer to total mass i.e. the sum of all the chemical species of the system. In the absence of a chemical reaction
the amount of any chemical species flowing in and out will be the same; This gives rise to an equation for each species in the system. However if this is not the case then the mass balance equation must be amended to allow for the generation or depletion (consumption) of each chemical species. Some use one term in this equation to account for chemical reactions, which will be negative for depletion and positive for generation. However, the conventional form of this equation is written to account for both a positive generation term (i.e. product of reaction) and a negative consumption term (the reactants used to produce the products). Although overall one term will account for the total balance on the system, if this balance equation is to be applied to an individual species and then the entire process, both terms are necessary. This modified equation can be used not only for reactive systems, but for population balances such as occur in particle mechanics problems. The equation is given below; Note that it simplifies to the earlier equation in the case that the generation term is zero.
 At this point a simple example shall be given for illustrative purposes. Consider the situation whereby a slurry
At this point a simple example shall be given for illustrative purposes. Consider the situation whereby a slurry
is flowing into a settling tank to remove the solids in the tank, solids are collected at the bottom by means of a conveyor belt
partially submerged in the tank, water exits via an overflow outlet.
In this example we shall consider there to be two species, solids and water. The species are concentrated in each of the output streams, that is to say that the water-to-solid ratio at the water-overflow outlet is higher than at the slurry inlet and the solids concentration at the exit of the conveyor belt is higher than that at the slurry inlet.
Assumptions
Analysis
The slurry inlet composition has been measured by sampling the inlet and has a composition (by mass) of 50% solid and 50% water, with a mass flow of 100 kg per minute, the tank is assumed to be operating at steady state, and as such accumulation is zero, so input and output must be equal for both the solids and water. If we know that the removal efficiency for the slurry tank is 60%, then the water outlet will contain 20kg/min of solids (40% times 100kg/min times 50% solids). If we measure the flow-rate of the combined solids and water, and the water outlet is shown to be 60kg/min, then the amount of water exiting via the conveyor belt is 10kg/min. This allows us to completely determine how the mass has been distributed in the system with only limited information and using the mass balance relations across the system boundaries
 Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit for often for further reprocessing.
Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit for often for further reprocessing.
Such systems are common in grinding circuits, where materials are crushed then sieved to only allow a particular size of particle out of the circuit and the larger particles are returned to the grinder. However recycle flows are by no means restricted to solid mechanics operations, they are used in liquid and gas flows as well. One such example is in cooling tower
s, where water is pumped through the cooling tower many times, with only a small quantity of water drawn off at each pass (to prevent solids build up) until it has either evaporated or exited with the drawn off water.
The use of the recycle aids in increasing overall conversion of input products, which is useful for low per-pass conversion processes, for example the Haber process
.
. The concept is the same as for a large mass balance, however it is performed in the context of a limiting system (for example, one can consider the limiting case in time or, more commonly, volume). The use of a differential mass balance is to generate differential equation
s that can be used to provide an understanding and effective modelling tool for the target system.
The differential mass balance is usually solved in two steps, firstly a set of governing differential equations must be obtained, and then these equations must be solved, either analytically or, for less tractable problems, numerically.
A good example of the applications of differential mass balance are shown in the following systems:
.
The mass balance for a substance A becomes
where rA denotes the rate at which substance A is produced, V is the volume (which may be constant or not), nA the number of moles (n) of substance A.
In a fed-batch reactor some reactants/ingredients are added continuously or in pulses (compare making porridge by either first blending all ingredients and the let it boil, which can be described as a batch reactor, or by first mixing only water and salt and making that boil before the other ingredients are added, which can be described as a fed-batch reactor). Mass balances for fed-batch reactors become a bit more complicated.
to derive the expression for a chemical equilibrium
constant.
Assume we have a closed reactor in which the following liquid phase reversible reaction occurs:
The mass balance for substance A becomes
As we have a liquid phase reaction we can (usually) assume a constant volume and since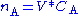 we get
we get
or
In many text books this is given as the definition of reaction rate
without specifying the implicit assumption that we are talking about reaction rate in a closed system with only one reaction. This is an unfortunate mistake that has confused many students over the years.
According to the law of mass action
the forward reaction rate can be written as
and the backward reaction rate as
The rate at which substance A is produced is thus
and since, at equilibrium, the concentration of A is constant we get
or, rearranged
where Q0 and Q denote the volumetric flow in and out of the system respectively and CA,0 and CA the concentration of A in the inflow and outflow respective. In an open system we can never reach a chemical equilibrium. We can, however, reach a steady state
where all state variables (temperature, concentrations etc.) remain constant ( )
)
Since there is no reaction, and since there is no outflow
and since there is no outflow 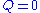 . The mass balance becomes
. The mass balance becomes
or
Using a mass balance for total volume, however, it is evident that and that
and that  . Thus we get
. Thus we get
Note that there is no reaction and hence no reaction rate
or rate law involved, and yet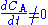 . We can thus draw the conclusion that reaction rate can not be defined in a general manner using
. We can thus draw the conclusion that reaction rate can not be defined in a general manner using 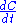 . One must first write down a mass balance before a link between
. One must first write down a mass balance before a link between  and the reaction rate can be found. Many textbooks, however, define reaction rate as
and the reaction rate can be found. Many textbooks, however, define reaction rate as
without mentioning that this definition implicitly assumes that the system is closed, has a constant volume and that there is only one reaction.
part of the tube and make a mass balance over that using the ideal tank reactor model. That mass balance is then integrated
over the entire reactor volume to obtain:
In numeric solutions, e.g. when using computers, the ideal tube is often translated to a series of tank reactors, as it can be shown that a PFR is equivalent to an infinite number of stirred tanks in series, but the latter is often easier to analyze, especially at steady state.
rates may be important in the mathematical description of a system, especially in heterogeneous systems.
As the chemical reaction rate
depends on temperature it is often necessary to make both an energy balance
(often a heat balance rather than a full fledged energy balance) as well as mass balances to fully describe the system. A different reactor models might be needed for the energy balance: A system that is closed with respect to mass might be open with respect to energy e.g. since heat may enter the system through conduction
.
algorithms may be employed to correct measured flows, provided that enough redundancy of flow measurements exist to permit statistical reconciliation and exclusion of detectably erroneous measurements. Since all real world measured values contain inherent error, the reconciled measurements provide a better basis than the measured values do for financial reporting, optimization, and regulatory reporting. Software packages exist to make this commercially feasible on a daily basis.
For example, the average precipitation over the Antarctic ice sheet is approximately 150 mm / year; the average ice depth is 3 km; therefore the average residence time of the ice within the ice sheet is approximately 20,000 years.
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
to the analysis of physical systems. By accounting for material entering and leaving a system, mass flow
Mass flow
Mass flow, also known as mass transfer and bulk flow, is the movement of material matter. In physics, mass flow occurs in open systems and is often measured as occurring when moving across a certain boundary characterized by its cross-sectional area and a flow rate. In engineering and biology it...
s can be identified which might have been unknown, or difficult to measure without this technique. The exact conservation law
Conservation law
In physics, a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves....
used in the analysis of the system depends on the context of the problem but all revolve around mass conservation, i.e. that matter
Matter
Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
cannot disappear or be created spontaneously.
Therefore, mass balances are used widely in engineering
Engineering
Engineering is the discipline, art, skill and profession of acquiring and applying scientific, mathematical, economic, social, and practical knowledge, in order to design and build structures, machines, devices, systems, materials and processes that safely realize improvements to the lives of...
and environmental analyses. For example mass balance theory is used to design chemical reactor
Chemical reactor
In chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
s, analyse alternative processes to produce chemicals as well as in pollution
Pollution
Pollution is the introduction of contaminants into a natural environment that causes instability, disorder, harm or discomfort to the ecosystem i.e. physical systems or living organisms. Pollution can take the form of chemical substances or energy, such as noise, heat or light...
dispersion models and other models of physical systems. Closely related and complementary analysis techniques include the population balance
Population balance equation
Population balance equations have been introduced in several branches of modern science, mainly in branches with particulate entities. This includes topics like crystallization, liquid-liquid extraction, gas-liquid dispersions, liquid-liquid reactions, communition, aerosol engineering, biology ,...
, energy balance
Energy accounting
Energy accounting is a system used within industry, where measuring and analyzing the energy consumption of different activities is done to improve energy efficiency.-Energy management:...
and the somewhat more complex entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
balance. These techniques are required for thorough design and analysis of systems such as the refrigeration cycle.
In environmental monitoring the term budget calculations is used to describe mass balance equations where they are used to evaluate the monitoring data (comparing input and output, etc.) In biology the dynamic energy budget
Dynamic energy budget
The Dynamic Energy Budget theory aims to identify simple quantitative rules for the organization of metabolism of individual organisms that can be understood from basic first principles...
theory for metabolic organisation makes explicit use of time, mass and energy balances.
Introduction
The general form quoted for a mass balance is The mass that enters a system must, by conservation of mass, either leave the system or accumulate within the system .Mathematically the mass balance for a system without a chemical reaction is as follows:
Strictly speaking the above equation holds also for systems with chemical reactions if the terms in the balance equation are taken to refer to total mass i.e. the sum of all the chemical species of the system. In the absence of a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
the amount of any chemical species flowing in and out will be the same; This gives rise to an equation for each species in the system. However if this is not the case then the mass balance equation must be amended to allow for the generation or depletion (consumption) of each chemical species. Some use one term in this equation to account for chemical reactions, which will be negative for depletion and positive for generation. However, the conventional form of this equation is written to account for both a positive generation term (i.e. product of reaction) and a negative consumption term (the reactants used to produce the products). Although overall one term will account for the total balance on the system, if this balance equation is to be applied to an individual species and then the entire process, both terms are necessary. This modified equation can be used not only for reactive systems, but for population balances such as occur in particle mechanics problems. The equation is given below; Note that it simplifies to the earlier equation in the case that the generation term is zero.
- In the absence of a nuclear reactionNuclear reactionIn nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce products different from the initial particles...
the number of atoms flowing in and out are the same, even in the presence of a chemical reaction - To perform a balance the boundaries of the system must be well defined
- Mass balances can be taken over physical systems at multiple scales.
- Mass balances can be simplified with the assumption of steady stateSteady stateA system in a steady state has numerous properties that are unchanging in time. This implies that for any property p of the system, the partial derivative with respect to time is zero:...
, where the accumulation term is zero
Illustrative example

Slurry
A slurry is, in general, a thick suspension of solids in a liquid.-Examples of slurries:Examples of slurries include:* Lahars* A mixture of water and cement to form concrete* A mixture of water, gelling agent, and oxidizers used as an explosive...
is flowing into a settling tank to remove the solids in the tank, solids are collected at the bottom by means of a conveyor belt
Conveyor belt
A conveyor belt consists of two or more pulleys, with a continuous loop of material - the conveyor belt - that rotates about them. One or both of the pulleys are powered, moving the belt and the material on the belt forward. The powered pulley is called the drive pulley while the unpowered pulley...
partially submerged in the tank, water exits via an overflow outlet.
In this example we shall consider there to be two species, solids and water. The species are concentrated in each of the output streams, that is to say that the water-to-solid ratio at the water-overflow outlet is higher than at the slurry inlet and the solids concentration at the exit of the conveyor belt is higher than that at the slurry inlet.
Assumptions
- Steady state
- Non-reactive system
Analysis
The slurry inlet composition has been measured by sampling the inlet and has a composition (by mass) of 50% solid and 50% water, with a mass flow of 100 kg per minute, the tank is assumed to be operating at steady state, and as such accumulation is zero, so input and output must be equal for both the solids and water. If we know that the removal efficiency for the slurry tank is 60%, then the water outlet will contain 20kg/min of solids (40% times 100kg/min times 50% solids). If we measure the flow-rate of the combined solids and water, and the water outlet is shown to be 60kg/min, then the amount of water exiting via the conveyor belt is 10kg/min. This allows us to completely determine how the mass has been distributed in the system with only limited information and using the mass balance relations across the system boundaries
Mass feedback (recycle)

Such systems are common in grinding circuits, where materials are crushed then sieved to only allow a particular size of particle out of the circuit and the larger particles are returned to the grinder. However recycle flows are by no means restricted to solid mechanics operations, they are used in liquid and gas flows as well. One such example is in cooling tower
Cooling tower
Cooling towers are heat removal devices used to transfer process waste heat to the atmosphere. Cooling towers may either use the evaporation of water to remove process heat and cool the working fluid to near the wet-bulb air temperature or in the case of closed circuit dry cooling towers rely...
s, where water is pumped through the cooling tower many times, with only a small quantity of water drawn off at each pass (to prevent solids build up) until it has either evaporated or exited with the drawn off water.
The use of the recycle aids in increasing overall conversion of input products, which is useful for low per-pass conversion processes, for example the Haber process
Haber process
The Haber process, also called the Haber–Bosch process, is the nitrogen fixation reaction of nitrogen gas and hydrogen gas, over an enriched iron or ruthenium catalyst, which is used to industrially produce ammonia....
.
Differential mass balances
A mass balance can also be taken differentiallyCalculus
Calculus is a branch of mathematics focused on limits, functions, derivatives, integrals, and infinite series. This subject constitutes a major part of modern mathematics education. It has two major branches, differential calculus and integral calculus, which are related by the fundamental theorem...
. The concept is the same as for a large mass balance, however it is performed in the context of a limiting system (for example, one can consider the limiting case in time or, more commonly, volume). The use of a differential mass balance is to generate differential equation
Differential equation
A differential equation is a mathematical equation for an unknown function of one or several variables that relates the values of the function itself and its derivatives of various orders...
s that can be used to provide an understanding and effective modelling tool for the target system.
The differential mass balance is usually solved in two steps, firstly a set of governing differential equations must be obtained, and then these equations must be solved, either analytically or, for less tractable problems, numerically.
A good example of the applications of differential mass balance are shown in the following systems:
- Ideal (stirred) Batch reactor
- Ideal tank reactor, also named Continuous Stirred Tank Reactor (CSTR)
- Ideal Plug Flow ReactorPlug flow reactor modelThe plug flow reactor model is used to describe chemical reactions in continuous, flowing systems. The PFR model is used to predict the behaviour of chemical reactors, so that key reactor variables, such as the dimensions of the reactor, can be estimated...
(PFR)
Ideal batch reactor
The ideal completely mixed batch reactor is a closed system. Isothermal conditions are assumed, and mixing prevents concentration gradients as reactant concentrations decrease and product concentrations increase over time. Many chemistry textbooks implicitly assume that the studied system can be described as a batch reactor when they write about reaction kinetics and chemical equilibriumChemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
.
The mass balance for a substance A becomes
where rA denotes the rate at which substance A is produced, V is the volume (which may be constant or not), nA the number of moles (n) of substance A.
In a fed-batch reactor some reactants/ingredients are added continuously or in pulses (compare making porridge by either first blending all ingredients and the let it boil, which can be described as a batch reactor, or by first mixing only water and salt and making that boil before the other ingredients are added, which can be described as a fed-batch reactor). Mass balances for fed-batch reactors become a bit more complicated.
Reactive example
In this example we will use the law of mass actionMass action
In Chemistry, the law of mass action is a mathematical model that explains and predicts behaviors of solutions in dynamic equilibrium. It can be described with two aspects: 1) the equilibrium aspect, concerning the composition of a reaction mixture at equilibrium and 2) the kinetic aspect...
to derive the expression for a chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
constant.
Assume we have a closed reactor in which the following liquid phase reversible reaction occurs:
The mass balance for substance A becomes
As we have a liquid phase reaction we can (usually) assume a constant volume and since
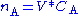 we get
we getor
In many text books this is given as the definition of reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
without specifying the implicit assumption that we are talking about reaction rate in a closed system with only one reaction. This is an unfortunate mistake that has confused many students over the years.
According to the law of mass action
Mass action
In Chemistry, the law of mass action is a mathematical model that explains and predicts behaviors of solutions in dynamic equilibrium. It can be described with two aspects: 1) the equilibrium aspect, concerning the composition of a reaction mixture at equilibrium and 2) the kinetic aspect...
the forward reaction rate can be written as
and the backward reaction rate as
The rate at which substance A is produced is thus
and since, at equilibrium, the concentration of A is constant we get
or, rearranged
Ideal tank reactor/continuously stirred tank reactor
The continuously mixed tank reactor is an open system with an influent stream of reactants and an effluent stream of products. A lake can be regarded as a tank reactor and lakes with long turnover times (e.g. with a low flux to volume ratio) can for many purposes be regarded as continuously stirred (e.g. homogeneous in all respects). The mass balance becomeswhere Q0 and Q denote the volumetric flow in and out of the system respectively and CA,0 and CA the concentration of A in the inflow and outflow respective. In an open system we can never reach a chemical equilibrium. We can, however, reach a steady state
Dynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
where all state variables (temperature, concentrations etc.) remain constant (
 )
)Example
Consider a bathtub in which there is some bathing salt dissolved. We now fill in more water, keeping the bottom plug in. What happens?Since there is no reaction,
 and since there is no outflow
and since there is no outflow 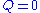 . The mass balance becomes
. The mass balance becomesor
Using a mass balance for total volume, however, it is evident that
 and that
and that  . Thus we get
. Thus we getNote that there is no reaction and hence no reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
or rate law involved, and yet
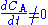 . We can thus draw the conclusion that reaction rate can not be defined in a general manner using
. We can thus draw the conclusion that reaction rate can not be defined in a general manner using 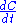 . One must first write down a mass balance before a link between
. One must first write down a mass balance before a link between  and the reaction rate can be found. Many textbooks, however, define reaction rate as
and the reaction rate can be found. Many textbooks, however, define reaction rate aswithout mentioning that this definition implicitly assumes that the system is closed, has a constant volume and that there is only one reaction.
Ideal plug flow reactor (PFR)
The idealized plug flow reactor is an open system resembling a tube with no mixing in the direction of flow but perfect mixing perpendicular to the direction of flow. Often used for systems like rivers and water pipes if the flow is turbulent. When a mass balance is made for a tube, one first considers an infinitesimalInfinitesimal
Infinitesimals have been used to express the idea of objects so small that there is no way to see them or to measure them. The word infinitesimal comes from a 17th century Modern Latin coinage infinitesimus, which originally referred to the "infinite-th" item in a series.In common speech, an...
part of the tube and make a mass balance over that using the ideal tank reactor model. That mass balance is then integrated
Integral
Integration is an important concept in mathematics and, together with its inverse, differentiation, is one of the two main operations in calculus...
over the entire reactor volume to obtain:
In numeric solutions, e.g. when using computers, the ideal tube is often translated to a series of tank reactors, as it can be shown that a PFR is equivalent to an infinite number of stirred tanks in series, but the latter is often easier to analyze, especially at steady state.
More complex problems
In reality, reactors are often non-ideal, in which combinations of the reactor models above are used to describe the system. Not only chemical reaction rates, but also mass transferMass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
rates may be important in the mathematical description of a system, especially in heterogeneous systems.
As the chemical reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
depends on temperature it is often necessary to make both an energy balance
Energy accounting
Energy accounting is a system used within industry, where measuring and analyzing the energy consumption of different activities is done to improve energy efficiency.-Energy management:...
(often a heat balance rather than a full fledged energy balance) as well as mass balances to fully describe the system. A different reactor models might be needed for the energy balance: A system that is closed with respect to mass might be open with respect to energy e.g. since heat may enter the system through conduction
Heat conduction
In heat transfer, conduction is a mode of transfer of energy within and between bodies of matter, due to a temperature gradient. Conduction means collisional and diffusive transfer of kinetic energy of particles of ponderable matter . Conduction takes place in all forms of ponderable matter, viz....
.
Commercial use
In industrial process plants, using the fact that the mass entering and leaving any portion of a process plant must balance, data validation and reconciliationData Validation and Reconciliation
Industrial process data validation and reconciliation or short data validation and reconciliation is a technology which is using process information and mathematical methods in order to automatically correct measurements in industrial processes...
algorithms may be employed to correct measured flows, provided that enough redundancy of flow measurements exist to permit statistical reconciliation and exclusion of detectably erroneous measurements. Since all real world measured values contain inherent error, the reconciled measurements provide a better basis than the measured values do for financial reporting, optimization, and regulatory reporting. Software packages exist to make this commercially feasible on a daily basis.
Mass balance of ice sheets
The mass balance concept can usefully be applied to ice sheets, which is of interest because of their relevance to sea level rise.For example, the average precipitation over the Antarctic ice sheet is approximately 150 mm / year; the average ice depth is 3 km; therefore the average residence time of the ice within the ice sheet is approximately 20,000 years.
See also
- BioreactorBioreactorA bioreactor may refer to any manufactured or engineered device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This...
- Chemical reactorChemical reactorIn chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
- Chemical engineeringChemical engineeringChemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
- Chemical equilibriumChemical equilibriumIn a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
- Conservation of massConservation of massThe law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
- Continuity equationContinuity equationA continuity equation in physics is a differential equation that describes the transport of a conserved quantity. Since mass, energy, momentum, electric charge and other natural quantities are conserved under their respective appropriate conditions, a variety of physical phenomena may be described...
- Continuous stirred-tank reactor
- Dilution (equation)Dilution (equation)Dilution is a reduction in the concentration of a chemical . It is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. To dilute a solution means to add more solvent without the addition of more solute...
- Energy accountingEnergy accountingEnergy accounting is a system used within industry, where measuring and analyzing the energy consumption of different activities is done to improve energy efficiency.-Energy management:...
- Mass actionMass actionIn Chemistry, the law of mass action is a mathematical model that explains and predicts behaviors of solutions in dynamic equilibrium. It can be described with two aspects: 1) the equilibrium aspect, concerning the composition of a reaction mixture at equilibrium and 2) the kinetic aspect...
- Mass fluxMass fluxMass flux is the rate of mass flow across a unit area .-See also:*Flux*Fick's law*Darcy's law...
- Material balance planningMaterial balance planningMaterial balance accounting is a form of economic accounting based on balancing inputs with outputs in terms of natural units...
- Data Validation and ReconciliationData Validation and ReconciliationIndustrial process data validation and reconciliation or short data validation and reconciliation is a technology which is using process information and mathematical methods in order to automatically correct measurements in industrial processes...