MO diagram
Encyclopedia
A molecular orbital diagram, or MO diagram for short, is a qualitative descriptive tool explaining chemical bonding in molecule
s in terms of molecular orbital theory
in general and the Linear combination of atomic orbitals molecular orbital method
(LCAO method) in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbital
s combine to form the same number of molecular orbital
s, although involved electron
s may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide
but becomes more complex when discussing polyatomic molecules such as methane
. It explains why some molecules exist and not others, how strong bonds are, and what electronic transitions can take place. In this article, MO stands for Molecular Orbital, and AO stands for Atomic Orbital.
and Friedrich Hund
. A mathematical description was provided by contributions from Douglas Hartree
in 1928 and Vladimir Fock
in 1930.
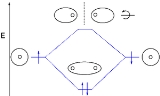
s, shown as short horizontal lines in the center, flanked on the sides by constituent AO energy levels for comparison, with the energy levels ranging from low energy at the bottom to high energy at the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Degenerate energy levels
are commonly shown side by side. Appropriate AO and MO levels are filled with electrons symbolized by small vertical arrows showing the electron spin. Pictures of the AO or MO shapes themselves are often not shown on these diagrams. For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO energies are shown on the sides as if the two atoms were free and unbonded. For simple polyatomic molecules with a "central atom" such as methane
(CH4) or carbon dioxide
(CO2), AO levels of the central atom may be shown on one side, and AO levels of the other atoms bonded to it shown on the other side, with the MO levels representing the bonds shown in the middle of the diagram. For other polyatomic molecules, an MO diagram may show merely a bond or some bonds of interest in the molecules, leaving others out for simplicity. Often even for simple molecules, AO and MO levels of inner orbitals and their electrons may be omitted from a diagram for simplicity.
In MO theory molecular orbitals form by overlap of atomic orbitals. The atomic orbital energy correlates with electronegativity
as a more electronegative atom holds an electron more tightly thus lowering its energy. MO treatment is only valid when the atomic orbitals have comparable energy; when they differ greatly the mode of bonding becomes ionic
. A second condition for mixing is that the atomic orbitals have the same symmetry.
The two atomic orbitals can overlap in two ways depending on their phase relationship. The phase of an orbital is a direct consequence of the wave-like properties of electrons. In graphical representations of orbitals, orbital phase is depicted either by a plus or minus sign (confusing because there is no relationship to electrical charge) or simply by shading one lobe. The sign of the phase itself does not have physical meaning except when mixing orbitals to form molecular orbitals.
Then two same-sign orbitals have a constructive overlap forming a molecular orbital with the bulk of electron density
located between the two nuclei. This MO is called the bonding orbital and its energy is lower than that of the original atomic orbitals. A bond involving molecular orbitals which are symmetrical with respect to rotation around the bond axis (no change) and is called a sigma bond
(σ-bond). In the event of a phase change, the bond becomes a pi bond
(π-bond). Symmetry labels are further defined by whether the orbital maintains its original character after an inversion about its center; if the orbital does retain its original character it is defined gerade
, g, or if the orbital does not maintain its original character, ungerade
, u.
Atomic orbitals can also interact with each other out-of-phase which leads to destructive cancellation and no electron density between the two nuclei at the so-called nodal plane depicted as a perpendicular dashed line. In this anti-bonding MO with energy much higher than the original AO's, any electrons present are located in lobes pointing away from the central internuclear axis. For a corresponding σ-bonding orbital, such an orbital would be symmetrical but differentiated from it by an asterisk
as in σ*. For a π-bond, corresponding bonding and antibonding orbitals would not have such symmetry around the bond axis and be designated π and π*, respectively.
The next step in constructing an MO diagram is filling the newly formed molecular orbitals with electrons. Three general rules apply:
The filled MO highest in energy is called the Highest Occupied Molecular Orbital
or HOMO and the empty MO just above it is then the Lowest Unoccupied Molecular Orbital
or LUMO. The electrons in the bonding MO's are called bonding electrons and any electrons in the antibonding orbital would be called antibonding electrons. The reduction in energy of these electrons is the driving force for chemical bond formation. Whenever mixing for an atomic orbital is not possible for reasons of symmetry or energy, a so-called non-bonding MO
is created, which is often quite similar to and has energy level equal or close to its constituent AO, thus not contributing to bonding energetics. The resulting electron configuration can be described in terms of bond type, parity and occupancy for example dihydrogen 1σg2. Alternatively it can be written as a molecular term symbol
e.g. 1Σ
g+ for dihydrogen. Sometimes, the letter n is used to designate a non-bonding orbital.
For a stable bond, the bond order
defined as:
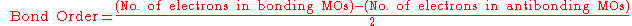
must be positive.
The relative order in MO energies and occupancy correspond with electronic transitions found in photoelectron spectroscopy (PES). In this way it is possible to experimentally verify MO theory. In general sharp PES transitions indicate nonbonding electrons and broad bands are indicative of bonding and antibonding delocalized electrons. Bands can resolve into fine structure with spacings corresponding to vibrational modes of the molecular cation (see Franck–Condon principle). PES energies are different from ionisation energies
which relates to the energy required to strip off the nth electron after the first n − 1 electrons have been removed. MO diagrams with energy values can be obtained mathematically using the Hartree–Fock method. The starting point for any MO diagram is a predefined molecular geometry
for the molecule in question. An exact relationship between geometry and orbital energies is given in Walsh diagram
s.
gas exists as dihydrogen (H-H) with a single covalent bond
between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital
for its electron
, the bond forms by overlap of these two atomic orbitals. In figure 1 the two atomic orbitals are depicted on the left and on the right. The vertical axis always represents the orbital energies. Each atomic orbital is singly occupied with an up or down arrow representing an electron.
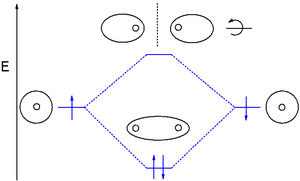 Application of MO theory for dihydrogen results in having both electrons in the bonding MO with electron configuration 1σg2. The bond order for dihydrogen is (2-0)/2 = 1. The photoelectron spectrum of dihydrogen shows a single set of multiplets between 16 and 18 eV
Application of MO theory for dihydrogen results in having both electrons in the bonding MO with electron configuration 1σg2. The bond order for dihydrogen is (2-0)/2 = 1. The photoelectron spectrum of dihydrogen shows a single set of multiplets between 16 and 18 eV
(electron volts).
The dihydrogen MO diagram helps explain how a bond breaks. When applying energy to dihydrogen, a molecular electronic transition
takes place when one electron in the bonding MO is promoted to the antibonding MO. The result is that there is no longer a net gain in energy.
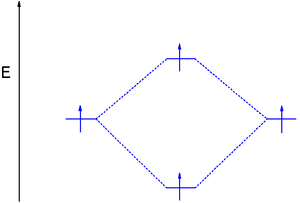
 The only way to accomplish this is by occupying the antibonding orbital with two electrons as well which reduces the bond order ((2-2)/2) to zero and cancels the net energy stabilization.
The only way to accomplish this is by occupying the antibonding orbital with two electrons as well which reduces the bond order ((2-2)/2) to zero and cancels the net energy stabilization.
Another molecule that is precluded based on this principle is diberyllium (beryllium
with electron configuration
1s22s2). On the other hand by removing one electron from dihelium, the stable gas-phase species He2+ ion is formed with bond order 1/2.
is lithium
and MO theory correctly predicts that dilithium
is a stable molecule with bond order 1 (configuration 1σg21σu22σg2). The 1s MOs are completely filled and do not participate in bonding.
 Dilithium is a gas-phase molecule with a much lower bond strength
Dilithium is a gas-phase molecule with a much lower bond strength
than dihydrogen because the 2s electrons are further removed from the nucleus. In a more detailed analysis both the 1σ orbitals have higher energies than the 1s AO and the occupied 2σ is also higher in energy than the 2s AO (see table 1).
boron
: 1s22s22p1) requires the introduction of an atomic orbital overlap model for p orbitals. The three dumbbell
-shaped p-orbitals have equal energy and are oriented mutually perpendicular (or orthogonal). The p-orbitals oriented in the x-direction (px) can overlap end-on forming a bonding (symmetrical) sigma orbital and an antibonding sigma* molecular orbital. In contrast to the sigma 1s MO's, the sigma 2p has some non-bonding electron density at either side of the nuclei and the sigma* 2p has some electron density between the nuclei.
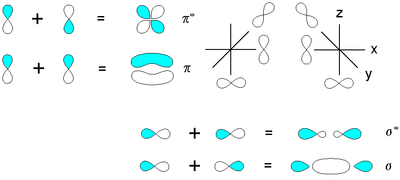 The other two p-orbitals py and pz can overlap side-on. The resulting bonding orbital has its electron density in the shape of two sausages above and below the plane of the molecule. The orbital is not symmetrical around the molecular axis and is therefore a pi orbital. The antibonding pi orbital (also asymmetrical) has four lobes pointing away from the nuclei. Both py and pz orbitals form a pair of pi orbitals equal in energy (degenerate
The other two p-orbitals py and pz can overlap side-on. The resulting bonding orbital has its electron density in the shape of two sausages above and below the plane of the molecule. The orbital is not symmetrical around the molecular axis and is therefore a pi orbital. The antibonding pi orbital (also asymmetrical) has four lobes pointing away from the nuclei. Both py and pz orbitals form a pair of pi orbitals equal in energy (degenerate
) and can be higher or lower than that of the sigma orbital.
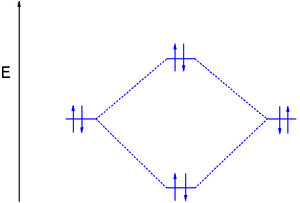
In diboron the 1s and 2s electrons do not participate in bonding but the single electrons in the 2p orbitals occupy the 2πpy and the 2πpz MO's resulting in bond order 1. Because the electrons have equal energy (they are degenerate) diboron is a diradical
and since the spins are parallel the compound is paramagnetic.
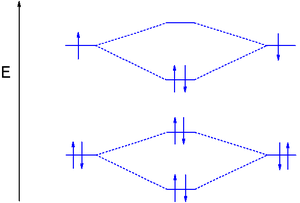
:1s22s22p2 MO's 2σg22σu21πu4) is a reactive gas-phase molecule. Two additional electrons are placed in the 2πp MO's increasing the bond order to 2.
(broad), the 2σg electrons at 37 eV (broad), the 2σu electrons at 19 eV (doublet), the 1πu4 electrons at 17 eV (multiplets), and finally the 3σg2 at 15.5 eV (sharp).
of 2. Just as diboron, when these unpaired electrons have the same spin, this type of dioxygen called triplet oxygen
is a paramagnetic diradical
. When both HOMO electrons pair up with opposite spins in one orbital, the other oxygen type is called singlet oxygen
.
 The bond order decreases and the bond length
The bond order decreases and the bond length
increases in the order O2+ (112.2 pm), O2 (121 pm), O2- (128 pm) and O22- (149 pm).
In difluorine
two additional electrons occupy the 2pπ* with a bond order of 1. In dineon Ne2 (as with dihelium) the number of bonding electrons equals the number of antibonding electrons and this compound does not exist.
values are similar. In carbon monoxide
(CO, isoelectronic with dinitrogen) the oxygen 2s orbital is much lower in energy than the carbon 2s orbital and therefore the degree of mixing is low. The electron configuration 1σ21σ*22σ22σ*21π43σ2 is identical to that of nitrogen. The g and u subscripts no longer apply because the molecule lacks a center of symmetry.
In hydrogen fluoride
(HF), the hydrogen 1s orbital can mix with fluorine 2pz orbital to form a sigma bond because experimentally the energy of 1s of hydrogen is comparable with 2p of fluorine. The HF electron configuration 1σ22σ23σ21π4 reflects that the other electrons remain in three lone pair
s and that the bond order is 1.
, CO2, is a linear molecule
with a total of sixteen bonding electrons
in its valence shell. Carbon is the central atom of the molecule and a principal axis, the z-axis, is visualized as a single axis that goes through the center of carbon and the two oxygens atoms.
For convention, blue atomic orbital lobes are positive phases, red atomic orbitals are negative phases, with respect to the wave function from the solution of the Schrödinger equation
. In carbon dioxide the carbon 2s (−19.4 eV), carbon 2p (−10.7 eV), and oxygen 2p (−15.9 eV)) energies associated with the atomic orbitals are in proximity whereas the oxygen 2s energy (−32.4 eV) is different.
Carbon and each oxygen atom will have a 2s atomic orbital and a 2p atomic orbital, where the p orbital is divided into px, py, and pz. With these derived atomic orbitals, symmetry labels are deduced with respect to rotation about the principal axis which generates a phase change, pi bond
(π) or generates no phase change, known as a sigma bond
(σ). Symmetry labels are further defined by whether the atomic orbital maintains its original character after an inversion about its center atom; if the atomic orbital does retain its original character it is defined gerade
,g, or if the atomic orbital does not maintain its original character, ungerade
, u. The final symmetry-labeled atomic orbital is now known as an irreducible representation.
Carbon dioxide’s molecular orbitals are made by the linear combination of atomic orbitals
of the same irreducible representation that are also similar in atomic orbital energy. Significant atomic orbital overlap is why sp bonding may occur. Strong mixing of the oxygen 2s atomic orbital is not to be expected and are non-bonding
degenerate
molecular orbitals. The combination of similar atomic orbital/wave functions and the combinations of atomic orbital/wave function inverses create particular energies associated with the nonbonding
(no change), bonding (lower than either parent orbital energy) and antibonding
(higher energy than either parent atomic orbital energy) molecular orbitals.
. The oxygen atomic orbitals are labeled according to their symmetry as a1 for the 2s2 orbital and b2, a1 and b2 for 4 electrons in the 2p orbital. The two hydrogen 1s orbitals are premixed to form a A1 (bonding) and B2 (antibonding) MO.
! C2v >
E
C2
σv(xz)
σv'(yz)
| A1 1
1
1
1
z
> x2, y2, z2
|-
| A2
1
1
−1
−1
Rz
xy
>-
| B1
1
−1
1
−1
x, Ry
xz
>-
| B2
1
−1
−1
1
y, Rx
yz
Mixing takes place between same-symmetry orbitals of comparable energy resulting a new set of MO's for water. The lowest-energy MO, 1a1 resembles the oxygen 2s AO with some mixing with the hydrogen A1 AO. Next is the 1b1 MO resulting from mixing of the oxygen b1 AO and the hydrogen B1 AO followed by the 2a1 MO created by mixing the a1 orbitals. Both MO's form the oxygen to hydrogen sigma bonds. The oxygen b2 AO (the p-orbital perpendicular to the molecular plane) alone forms the 1b2 MO is it is unable to mix. This MO is nonbonding. In agreement with this description the photoelectron spectrum for water shows two broad peaks for the 1b2 MO (18.5 eV) and the 2a1 MO (14.5 eV) and a sharp peak for the nonbonding 1b1 MO at 12.5 eV. This MO treatment of water differs from the orbital hybridisation
picture because now the oxygen atom has just one lone pair instead of two. In this sense, water does not have two equivalent lone electron pairs resembling rabbit ears.
Hydrogen sulfide
(H2S) too has a C2v symmetry with 8 valence electrons but the bending angle is only 92°. As reflected in its PE spectrum as compared to water the 2a1 MO is stabilised (improved overlap) and the 1b2 MO is destabilized (poorer overlap).
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s in terms of molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
in general and the Linear combination of atomic orbitals molecular orbital method
Linear combination of atomic orbitals molecular orbital method
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunctions...
(LCAO method) in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s combine to form the same number of molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s, although involved electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
but becomes more complex when discussing polyatomic molecules such as methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
. It explains why some molecules exist and not others, how strong bonds are, and what electronic transitions can take place. In this article, MO stands for Molecular Orbital, and AO stands for Atomic Orbital.
History
Qualitative MO theory was introduced in 1928 by Robert S. MullikenRobert S. Mulliken
Robert Sanderson Mulliken was an American physicist and chemist, primarily responsible for the early development of molecular orbital theory, i.e. the elaboration of the molecular orbital method of computing the structure of molecules. Dr. Mulliken received the Nobel Prize for chemistry in 1966...
and Friedrich Hund
Friedrich Hund
Friedrich Hermann Hund was a German physicist from Karlsruhe known for his work on atoms and molecules.Hund worked at the Universities of Rostock, Leipzig, Jena, Frankfurt am Main, and Göttingen....
. A mathematical description was provided by contributions from Douglas Hartree
Douglas Hartree
Douglas Rayner Hartree PhD, FRS was an English mathematician and physicist most famous for the development of numerical analysis and its application to the Hartree-Fock equations of atomic physics and the construction of the meccano differential analyser.-Early life:Douglas Hartree was born in...
in 1928 and Vladimir Fock
Vladimir Fock
Vladimir Aleksandrovich Fock was a Soviet physicist, who did foundational work on quantum mechanics and quantum electrodynamics....
in 1930.
Basics
Molecular orbital diagrams are diagrams of MO energy levelEnergy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
s, shown as short horizontal lines in the center, flanked on the sides by constituent AO energy levels for comparison, with the energy levels ranging from low energy at the bottom to high energy at the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Degenerate energy levels
Degenerate energy levels
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
are commonly shown side by side. Appropriate AO and MO levels are filled with electrons symbolized by small vertical arrows showing the electron spin. Pictures of the AO or MO shapes themselves are often not shown on these diagrams. For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO energies are shown on the sides as if the two atoms were free and unbonded. For simple polyatomic molecules with a "central atom" such as methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
(CH4) or carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2), AO levels of the central atom may be shown on one side, and AO levels of the other atoms bonded to it shown on the other side, with the MO levels representing the bonds shown in the middle of the diagram. For other polyatomic molecules, an MO diagram may show merely a bond or some bonds of interest in the molecules, leaving others out for simplicity. Often even for simple molecules, AO and MO levels of inner orbitals and their electrons may be omitted from a diagram for simplicity.
In MO theory molecular orbitals form by overlap of atomic orbitals. The atomic orbital energy correlates with electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
as a more electronegative atom holds an electron more tightly thus lowering its energy. MO treatment is only valid when the atomic orbitals have comparable energy; when they differ greatly the mode of bonding becomes ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
. A second condition for mixing is that the atomic orbitals have the same symmetry.
| MO diagram for dihydrogen. Here electrons are shown by dots. |
|---|
The two atomic orbitals can overlap in two ways depending on their phase relationship. The phase of an orbital is a direct consequence of the wave-like properties of electrons. In graphical representations of orbitals, orbital phase is depicted either by a plus or minus sign (confusing because there is no relationship to electrical charge) or simply by shading one lobe. The sign of the phase itself does not have physical meaning except when mixing orbitals to form molecular orbitals.
Then two same-sign orbitals have a constructive overlap forming a molecular orbital with the bulk of electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
located between the two nuclei. This MO is called the bonding orbital and its energy is lower than that of the original atomic orbitals. A bond involving molecular orbitals which are symmetrical with respect to rotation around the bond axis (no change) and is called a sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
(σ-bond). In the event of a phase change, the bond becomes a pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
(π-bond). Symmetry labels are further defined by whether the orbital maintains its original character after an inversion about its center; if the orbital does retain its original character it is defined gerade
Molecular term symbol
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term...
, g, or if the orbital does not maintain its original character, ungerade
Molecular term symbol
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term...
, u.
Atomic orbitals can also interact with each other out-of-phase which leads to destructive cancellation and no electron density between the two nuclei at the so-called nodal plane depicted as a perpendicular dashed line. In this anti-bonding MO with energy much higher than the original AO's, any electrons present are located in lobes pointing away from the central internuclear axis. For a corresponding σ-bonding orbital, such an orbital would be symmetrical but differentiated from it by an asterisk
Asterisk
An asterisk is a typographical symbol or glyph. It is so called because it resembles a conventional image of a star. Computer scientists and mathematicians often pronounce it as star...
as in σ*. For a π-bond, corresponding bonding and antibonding orbitals would not have such symmetry around the bond axis and be designated π and π*, respectively.
The next step in constructing an MO diagram is filling the newly formed molecular orbitals with electrons. Three general rules apply:
- The Aufbau principleAufbau principleThe Aufbau principle is used to determine the electron configuration of an atom, molecule or ion. The principle postulates a hypothetical process in which an atom is "built up" by progressively adding electrons...
states that orbitals are filled starting with the lowest energy - The Pauli exclusion principlePauli exclusion principleThe Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles...
states that the maximum number of electrons occupying an orbital is two having opposite spins - Hund's rule states that when there are several MO's with equal energy, the electrons fill into the MO's one at a time before filling two electrons into any.
The filled MO highest in energy is called the Highest Occupied Molecular Orbital
HOMO/LUMO
HOMO and LUMO are acronyms for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap...
or HOMO and the empty MO just above it is then the Lowest Unoccupied Molecular Orbital
HOMO/LUMO
HOMO and LUMO are acronyms for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap...
or LUMO. The electrons in the bonding MO's are called bonding electrons and any electrons in the antibonding orbital would be called antibonding electrons. The reduction in energy of these electrons is the driving force for chemical bond formation. Whenever mixing for an atomic orbital is not possible for reasons of symmetry or energy, a so-called non-bonding MO
Non-bonding orbital
A non-bonding orbital, also known as non-bonding molecular orbital and sometimes designated by the letter n in molecular orbital diagrams, is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms...
is created, which is often quite similar to and has energy level equal or close to its constituent AO, thus not contributing to bonding energetics. The resulting electron configuration can be described in terms of bond type, parity and occupancy for example dihydrogen 1σg2. Alternatively it can be written as a molecular term symbol
Molecular term symbol
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term...
e.g. 1Σ
Sigma
Sigma is the eighteenth letter of the Greek alphabet, and carries the 'S' sound. In the system of Greek numerals it has a value of 200. When used at the end of a word, and the word is not all upper case, the final form is used, e.g...
g+ for dihydrogen. Sometimes, the letter n is used to designate a non-bonding orbital.
For a stable bond, the bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
defined as:
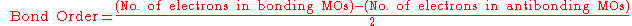
must be positive.
The relative order in MO energies and occupancy correspond with electronic transitions found in photoelectron spectroscopy (PES). In this way it is possible to experimentally verify MO theory. In general sharp PES transitions indicate nonbonding electrons and broad bands are indicative of bonding and antibonding delocalized electrons. Bands can resolve into fine structure with spacings corresponding to vibrational modes of the molecular cation (see Franck–Condon principle). PES energies are different from ionisation energies
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
which relates to the energy required to strip off the nth electron after the first n − 1 electrons have been removed. MO diagrams with energy values can be obtained mathematically using the Hartree–Fock method. The starting point for any MO diagram is a predefined molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
for the molecule in question. An exact relationship between geometry and orbital energies is given in Walsh diagram
Walsh diagram
Walsh diagrams, often called angular coordinate diagrams or correlation diagrams, are representations of calculated orbital energies of a molecule versus a distortion coordinate, used for making quick predictions about the geometries of small molecules...
s.
Dihydrogen MO diagram
The smallest molecule, hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas exists as dihydrogen (H-H) with a single covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
for its electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
, the bond forms by overlap of these two atomic orbitals. In figure 1 the two atomic orbitals are depicted on the left and on the right. The vertical axis always represents the orbital energies. Each atomic orbital is singly occupied with an up or down arrow representing an electron.

Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
(electron volts).
The dihydrogen MO diagram helps explain how a bond breaks. When applying energy to dihydrogen, a molecular electronic transition
Molecular electronic transition
Molecular electronic transitions take place when electrons in a molecule are excited from one energy level to a higher energy level. The energy change associated with this transition provides information on the structure of a molecule and determines many molecular properties such as color...
takes place when one electron in the bonding MO is promoted to the antibonding MO. The result is that there is no longer a net gain in energy.

Dihelium MO diagram
Dihelium (He-He) is a hypothetical molecule and MO theory helps to explain why dihelium does not exist in nature. The MO diagram for dihelium (2 electrons in each 1s AO) looks very similar to that of dihydrogen but instead of 2 electrons it is now required to place 4 electrons in the newly formed molecular orbitals.
Another molecule that is precluded based on this principle is diberyllium (beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
with electron configuration
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
1s22s2). On the other hand by removing one electron from dihelium, the stable gas-phase species He2+ ion is formed with bond order 1/2.
Dilithium MO diagram
Next up in the periodic tablePeriodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
is lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
and MO theory correctly predicts that dilithium
Dilithium
Dilithium, Li2, is a diatomic molecule comprising two lithium atoms covalently bonded together. Li2 is known in the gas phase. It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy of 101 kJ mol−1....
is a stable molecule with bond order 1 (configuration 1σg21σu22σg2). The 1s MOs are completely filled and do not participate in bonding.

Bond strength
In chemistry, bond strength is measured between two atoms joined in a chemical bond. It is the degree to which each atom linked to another atom contributes to the valency of this other atom...
than dihydrogen because the 2s electrons are further removed from the nucleus. In a more detailed analysis both the 1σ orbitals have higher energies than the 1s AO and the occupied 2σ is also higher in energy than the 2s AO (see table 1).
Diboron MO diagram
The MO diagram for diboron (B-B electron configurationElectron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
: 1s22s22p1) requires the introduction of an atomic orbital overlap model for p orbitals. The three dumbbell
Dumbbell
The dumbbell, a type of free weight, is a piece of equipment used in weight training. It can be used individually or in pairs .-History:...
-shaped p-orbitals have equal energy and are oriented mutually perpendicular (or orthogonal). The p-orbitals oriented in the x-direction (px) can overlap end-on forming a bonding (symmetrical) sigma orbital and an antibonding sigma* molecular orbital. In contrast to the sigma 1s MO's, the sigma 2p has some non-bonding electron density at either side of the nuclei and the sigma* 2p has some electron density between the nuclei.
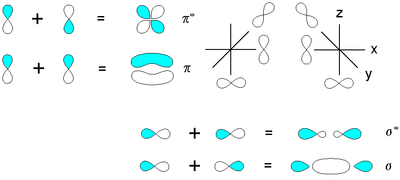
Degenerate energy level
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
) and can be higher or lower than that of the sigma orbital.
In diboron the 1s and 2s electrons do not participate in bonding but the single electrons in the 2p orbitals occupy the 2πpy and the 2πpz MO's resulting in bond order 1. Because the electrons have equal energy (they are degenerate) diboron is a diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
and since the spins are parallel the compound is paramagnetic.

Dicarbon MO diagram
Like diboron, dicarbon (C-C electron configurationElectron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
:1s22s22p2 MO's 2σg22σu21πu4) is a reactive gas-phase molecule. Two additional electrons are placed in the 2πp MO's increasing the bond order to 2.
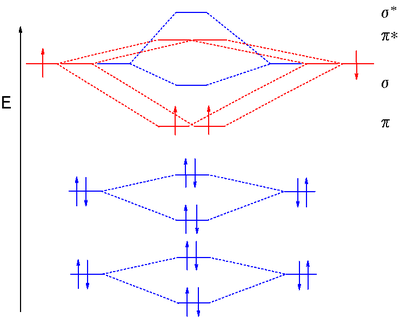
Dinitrogen MO diagram
The bond order for dinitrogen (2σg22σu21πu43σg2) is three because now two electrons are added in the 3σ MO as well. The MO diagram correlates with the experimental photoelectron spectrum for nitrogen. The 1σ electrons can be matched to a peak at 410 eVElectronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
(broad), the 2σg electrons at 37 eV (broad), the 2σu electrons at 19 eV (doublet), the 1πu4 electrons at 17 eV (multiplets), and finally the 3σg2 at 15.5 eV (sharp).
Dioxygen MO diagram
MO treatment of dioxygen is different from that of the previous diatomic molecules because the pσ MO is now lower in energy than the 2π orbitals. This is attributed to interaction between the 2s MO and the 2pz MO. Distributing 8 electrons over 6 molecular orbitals leaves the final two electrons as a degenerate pair in the 2pπ* antibonding orbitals resulting in a bond orderBond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
of 2. Just as diboron, when these unpaired electrons have the same spin, this type of dioxygen called triplet oxygen
Triplet oxygen
Triplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
is a paramagnetic diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
. When both HOMO electrons pair up with opposite spins in one orbital, the other oxygen type is called singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
.

Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
increases in the order O2+ (112.2 pm), O2 (121 pm), O2- (128 pm) and O22- (149 pm).
In difluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
two additional electrons occupy the 2pπ* with a bond order of 1. In dineon Ne2 (as with dihelium) the number of bonding electrons equals the number of antibonding electrons and this compound does not exist.
MO energies overview
Table 1 gives an overview of MO energies for first row diatomic molecules together with atomic orbital energies.| Table 1. Calculated MO energies for diatomic molecules in Hartrees | |||||||
|---|---|---|---|---|---|---|---|
| H2 | Li2 | B2 | C2 | N2 | O2 | F2 | |
| 1σg | -0.5969 | -2.4523 | -7.7040 | - 11.3598 | - 15.6820 | - 20.7296 | -26.4289 |
| 1σu | -2.4520 | -7.7032 | -11.3575 | -15.6783 | -20.7286 | -26.4286 | |
| 2σg | -0.1816 | -0.7057 | |||||
| -1.6488 | -1.7620 | ||||||
| 2σu | |||||||
| -0.5172 | -0.7780 | -1.0987 | -1.4997 | ||||
| 3σg | -0.6350 | -0.7358 | -0.7504 | ||||
| 1πu | |||||||
| -0.4579 | |||||||
| 1πg | |||||||
| -0.6682 | |||||||
| 1s (AO) | -0.5 | -2.4778 | -7.6953 | -11.3255 | -15.6289 | -20.6686 | -26.3829 |
| 2s (AO) | -0.1963 | ||||||
| -0.7056 | |||||||
| -1.2443 | -1.5726 | ||||||
| 2p (AO) | -0.3099 | -0.4333 | -0.5677 | -0.6319 | |||
Heteronuclear diatomics
In heteronuclear diatomic molecules, mixing of atomic orbitals only occurs when the electronegativityElectronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
values are similar. In carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(CO, isoelectronic with dinitrogen) the oxygen 2s orbital is much lower in energy than the carbon 2s orbital and therefore the degree of mixing is low. The electron configuration 1σ21σ*22σ22σ*21π43σ2 is identical to that of nitrogen. The g and u subscripts no longer apply because the molecule lacks a center of symmetry.
In hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
(HF), the hydrogen 1s orbital can mix with fluorine 2pz orbital to form a sigma bond because experimentally the energy of 1s of hydrogen is comparable with 2p of fluorine. The HF electron configuration 1σ22σ23σ21π4 reflects that the other electrons remain in three lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s and that the bond order is 1.
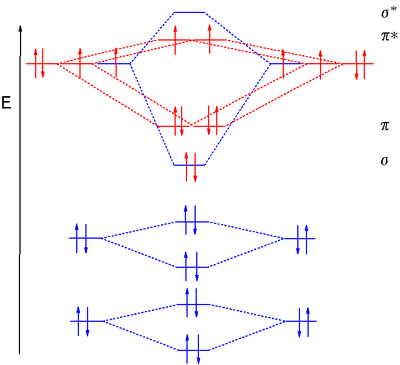
Carbon Dioxide MO Diagram
Carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, CO2, is a linear molecule
Linear molecular geometry
In chemistry, the Linear molecular geometry describes the arrangement of three or more atoms placed at an expected bond angle of 180º. Linear organic molecules, e.g. acetylene, are often described by invoking sp orbital hybridization for the carbon centers. Many linear molecules exist, prominent...
with a total of sixteen bonding electrons
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
in its valence shell. Carbon is the central atom of the molecule and a principal axis, the z-axis, is visualized as a single axis that goes through the center of carbon and the two oxygens atoms.
For convention, blue atomic orbital lobes are positive phases, red atomic orbitals are negative phases, with respect to the wave function from the solution of the Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
. In carbon dioxide the carbon 2s (−19.4 eV), carbon 2p (−10.7 eV), and oxygen 2p (−15.9 eV)) energies associated with the atomic orbitals are in proximity whereas the oxygen 2s energy (−32.4 eV) is different.
Carbon and each oxygen atom will have a 2s atomic orbital and a 2p atomic orbital, where the p orbital is divided into px, py, and pz. With these derived atomic orbitals, symmetry labels are deduced with respect to rotation about the principal axis which generates a phase change, pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
(π) or generates no phase change, known as a sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
(σ). Symmetry labels are further defined by whether the atomic orbital maintains its original character after an inversion about its center atom; if the atomic orbital does retain its original character it is defined gerade
Molecular term symbol
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term...
,g, or if the atomic orbital does not maintain its original character, ungerade
Molecular term symbol
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term...
, u. The final symmetry-labeled atomic orbital is now known as an irreducible representation.
Carbon dioxide’s molecular orbitals are made by the linear combination of atomic orbitals
Linear combination of atomic orbitals molecular orbital method
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunctions...
of the same irreducible representation that are also similar in atomic orbital energy. Significant atomic orbital overlap is why sp bonding may occur. Strong mixing of the oxygen 2s atomic orbital is not to be expected and are non-bonding
Non-bonding orbital
A non-bonding orbital, also known as non-bonding molecular orbital and sometimes designated by the letter n in molecular orbital diagrams, is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms...
degenerate
Degenerate energy levels
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
molecular orbitals. The combination of similar atomic orbital/wave functions and the combinations of atomic orbital/wave function inverses create particular energies associated with the nonbonding
Non-bonding orbital
A non-bonding orbital, also known as non-bonding molecular orbital and sometimes designated by the letter n in molecular orbital diagrams, is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms...
(no change), bonding (lower than either parent orbital energy) and antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
(higher energy than either parent atomic orbital energy) molecular orbitals.
Water MO diagram
Water (H2O) is a bent molecule (105°) with C2v molecular symmetryMolecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
. The oxygen atomic orbitals are labeled according to their symmetry as a1 for the 2s2 orbital and b2, a1 and b2 for 4 electrons in the 2p orbital. The two hydrogen 1s orbitals are premixed to form a A1 (bonding) and B2 (antibonding) MO.
| A1
> x2, y2, z2
|-
| A2
>-
| B1
>-
| B2
Mixing takes place between same-symmetry orbitals of comparable energy resulting a new set of MO's for water. The lowest-energy MO, 1a1 resembles the oxygen 2s AO with some mixing with the hydrogen A1 AO. Next is the 1b1 MO resulting from mixing of the oxygen b1 AO and the hydrogen B1 AO followed by the 2a1 MO created by mixing the a1 orbitals. Both MO's form the oxygen to hydrogen sigma bonds. The oxygen b2 AO (the p-orbital perpendicular to the molecular plane) alone forms the 1b2 MO is it is unable to mix. This MO is nonbonding. In agreement with this description the photoelectron spectrum for water shows two broad peaks for the 1b2 MO (18.5 eV) and the 2a1 MO (14.5 eV) and a sharp peak for the nonbonding 1b1 MO at 12.5 eV. This MO treatment of water differs from the orbital hybridisation
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
picture because now the oxygen atom has just one lone pair instead of two. In this sense, water does not have two equivalent lone electron pairs resembling rabbit ears.
Hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
(H2S) too has a C2v symmetry with 8 valence electrons but the bending angle is only 92°. As reflected in its PE spectrum as compared to water the 2a1 MO is stabilised (improved overlap) and the 1b2 MO is destabilized (poorer overlap).

