
MHC class I
Encyclopedia
Major histocompatibility complex
Major histocompatibility complex is a cell surface molecule encoded by a large gene family in all vertebrates. MHC molecules mediate interactions of leukocytes, also called white blood cells , which are immune cells, with other leukocytes or body cells...
(MHC) molecules (the other one being MHC class II
MHC class II
MHC Class II molecules are found only on a few specialized cell types, including macrophages, dendritic cells and B cells, all of which are professional antigen-presenting cells ....
) and are found on every nucleated cell of the body (and thus not on red blood cells, though they are found on platelets). Their function is to display fragments of proteins from within the cell to T cell
T cell
T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B cells and natural killer cells , by the presence of a T cell receptor on the cell surface. They are...
s; healthy cells will be ignored, while cells containing foreign proteins will be attacked by the immune system. Because MHC class I molecules present peptides derived from cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
ic proteins, the pathway of MHC class I presentation is often called the cytosolic or endogenous pathway.
Function
Class I MHC molecules bind peptides generated mainly from degradation of cytosolic proteins by the proteasomeProteasome
Proteasomes are very large protein complexes inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, they are located in the nucleus and the cytoplasm. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks...
. The MHC I:peptide complex is then inserted into the plasma membrane of the cell. The peptide is bound to the extracellular part of the class I MHC molecule. Thus, the function of the class I MHC is to display intracellular proteins to cytotoxic T cell
Cytotoxic T cell
A cytotoxic T cell belongs to a sub-group of T lymphocytes that are capable of inducing the death of infected somatic or tumor cells; they kill cells that are infected with viruses , or are otherwise damaged or...
s (CTLs). However, class I MHC can also present peptides generated from exogenous proteins, in a process known as cross-presentation
Cross-presentation
The term cross-presentation denotes the ability of certain antigen-presenting cells to take up, process and present extracellular antigens with MHC class I molecules to CD8 T cells . Cross-priming describes the stimulation of naive cytotoxic CD8+ T cells by this process...
.
A normal cell will display peptides from normal cellular protein turnover on its class I MHC, and CTLs will not be activated in response to them due to central and peripheral tolerance mechanisms. When a cell expresses foreign proteins, such as after viral infection, a fraction of the class I MHC will display these peptides on the cell surface. Consequently, CTLs specific for the MHC:peptide complex will recognize and kill the presenting cell.
Alternatively, class I MHC itself can serve as an inhibitory ligand for natural killer cells (NKs). Reduction in the normal levels of surface class I MHC, a mechanism employed by some viruses during immune evasion or in certain tumors, will activate NK cell killing.
Structure
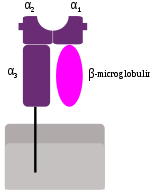
MHC class I molecules consist of two polypeptide chains, α and β2-microglobulin (b2m). The two chains are linked noncovalently via interaction of b2m and the α3 domain. Only the α chain is polymorphic and encoded by a HLA gene, while the b2m subunit is not polymorphic and encoded by the Beta-2 microglobulinBeta-2 microglobulin
β2 microglobulin also known as B2M is a component of MHC class I molecules, which are present on all nucleated cells . In humans, the β2 microglobulin protein is encoded by the B2M gene.-Structure and function:...
gene. The α3 domain is plasma membrane-spanning and interacts with the CD8
CD8
CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor . Like the TCR, CD8 binds to a major histocompatibility complex molecule, but is specific for the class I MHC protein. There are two isoforms of the protein, alpha and beta, each encoded by a different gene...
co-receptor of T-cells
T cell
T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B cells and natural killer cells , by the presence of a T cell receptor on the cell surface. They are...
. The α1 and α2 domains fold to make up a groove for peptides to bind. MHC class I molecules bind peptides that are 8-10 amino acid in length (Parham 87).
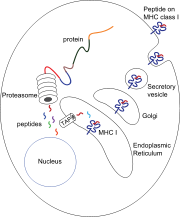
Production
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
by the proteasome
Proteasome
Proteasomes are very large protein complexes inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, they are located in the nucleus and the cytoplasm. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks...
. The proteasome is a macromolecule that consists of 28 subunits, of which half affect proteolytic
Proteolysis
Proteolysis is the directed degradation of proteins by cellular enzymes called proteases or by intramolecular digestion.-Purposes:Proteolysis is used by the cell for several purposes...
activity. The proteasome degrades intracellular proteins into small peptides that are then released into the cytosol. The peptides have to be translocated from the cytosol into the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
(ER) to meet the MHC class I molecule, whose peptide-binding site is in the lumen of the ER.
They have membrane proximal Ig fold.
Translocation and peptide loading
The peptide translocation from the cytosol into the lumen of the ER is accomplished by the transporter associated with antigen processingTransporter associated with antigen processing
Transporter associated with antigen processing is a member of the ATP-binding-cassette transporter family. It delivers cytosolic peptides into the endoplasmic reticulum , where they bind to nascent MHC class I molecules....
(TAP). TAP is a member of the ABC transporter family and is a heterodimeric multimembrane-spanning polypeptide consisting of TAP1 and TAP2. The two subunits form a peptide binding site and two ATP binding sites that face the lumen of the cytosol. TAP binds peptides on the cytoplasmic site and translocates them under ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
consumption into the lumen of the ER. The MHC class I molecule is then, in turn, loaded with peptides in the lumen of the ER.
The peptide-loading process involves several other molecules that form a large multimeric complex consisting of TAP, tapasin
Tapasin
TAP-associated glycoprotein also known as tapasin or TAPBP is a protein that in humans is encoded by the TAPBP gene.- Function :...
, calreticulin
Calreticulin
Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 is a protein that in humans is encoded by the CALR gene....
, calnexin
Calnexin
Calnexin is a 90kDa integral protein of the endoplasmic reticulum . It consists of a large N-terminal calcium-binding lumenal domain, a single transmembrane helix and a short , acidic cytoplasmic tail....
, and Erp57. Calnexin acts to stabilize the class I MHC α chains prior to β2m binding. Following complete assembly of the MHC molecule, calnexin dissociates. The MHC molecule lacking a bound peptide is inherently unstable and requires the binding of the chaperones calreticulin and Erp57. Additionally, tapasin binds to the MHC molecule and serves to link it to the TAP proteins, thus facilitating enhanced peptide loading and colocalization.
Once the peptide is loaded onto the MHC class I molecule, the complex dissociates and it leaves the ER through the secretory pathway
Secretory pathway
The secretory pathway is a series of steps a cell uses to move proteins out of the cell; a process known as secretion. The path of a protein destined for secretion has its origins in the rough endoplasmic reticulum, a membrane-bound compartment in the cell...
to reach the cell surface. The transport of the MHC class I molecules through the secretory pathway involves several posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
s of the MHC molecule. Some of the posttranslational modifications occur in the ER and involve change to the N-glycan regions of the protein, followed by extensive changes to the N-glycans in the Golgi apparatus
Golgi apparatus
The Golgi apparatus is an organelle found in most eukaryotic cells. It was identified in 1898 by the Italian physician Camillo Golgi, after whom the Golgi apparatus is named....
. The N-glycans mature fully before they reach the cell surface.
Peptide removal
Peptides that fail to bind MHC class I molecules in the lumen of the endoplasmic reticulum (ER) are removed from the ER via the sec61Sec61
Sec61 is an endoplasmic reticulum membrane protein translocator . It is a doughnut shaped pore through the membrane with 3 major subunits . It has a region called the plug that blocks transport into or out of the ER...
channel into the cytosol, where they might undergo further trimming in size, and might be translocated by TAP back into ER for binding to an MHC class I molecule.
For example, an interaction of sec61 with bovine albumin
Albumin
Albumin refers generally to any protein that is water soluble, which is moderately soluble in concentrated salt solutions, and experiences heat denaturation. They are commonly found in blood plasma, and are unique to other blood proteins in that they are not glycosylated...
has been observed.
Effect of viruses
MHC class I molecules are loaded with peptides generated from the degradation of ubiquitinated cytosolic proteins in proteasomes. As viruses induce cellular expression of viral proteins, some of these products are tagged for degradation, with the resulting peptide fragments entering the endoplasmic reticulum and binding to MHC I molecules. It is in this way, the MHC class I - dependent pathway of antigen presentation, that the virus infected cells signal T-Cells that abnormal proteins are being produced as a result of infectionThe fate of the virus-infected cell is almost always induction of apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
through cell-mediated immunity
Cell-mediated immunity
Cell-mediated immunity is an immune response that does not involve antibodies but rather involves the activation of macrophages, natural killer cells , antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen...
, reducing the risk of infecting neighboring cells. As an evolutionary response to this method of immune surveillance, many viruses are able to down-regulate or otherwise prevent the presentation of MHC class I molecules on the cell surface. In contrast to cytotoxic T lymphocytes, Natural killer (NK) cells are normally inactivated upon recognizing MHC I molecules on the surface of cells. Therefore, in the absence of MHC I molecules, NK cells are activated and recognize the cell as aberrant, suggesting they may be infected by viruses attempting to evade immune destruction. Several human cancers also show down-regulation of MHC I, giving transformed cells the same survival advantage of being able to avoid normal immune surveillance designed to destroy any infected or transformed cells.
Genes and isotypes
- Very polymorphic
- (HLA-AHLA-AHLA-A are a group of human leukocyte antigens that are encoded by the HLA-A locus on human chromosome 6p. The HLA genes constitute a large subset of the Major histocompatibility complex of humans. HLA-A is a component of certain MHC class I cell surface receptor isoforms that resides on the...
) - (HLA-BHLA-BHLA-B is a human gene that provides instructions for making a protein that plays a critical role in the immune system. HLA-B is part of a family of genes called the human leukocyte antigen complex...
) - (HLA-CHLA-CHLA-C belongs to the MHC class I heavy chain receptors. The C receptor is a heterodimer consisting of a HLA-C mature gene product and β2-microglobulin. The mature C chain is anchored in the membrane...
)
- (HLA-A
- Less polymorphic
- (HLA-E)
- (HLA-FHLA-FHLA class I histocompatibility antigen, alpha chain F is a protein that in humans is encoded by the HLA-F gene.-Further reading:...
) - (HLA-GHLA-GHLA-G histocompatibility antigen, class I, G, also known as human leukocyte antigen G , is a protein that in humans is encoded by the HLA-G gene....
)

