
Lightstick
Encyclopedia

Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
tube containing isolated substances which when combined make light through a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
-induced chemiluminescence which does not require an electrical power source. Although the glow stick is often used for recreation, it may also be relied upon for light during important military, police and fire, or EMS operations.
History
Cyalume was invented by Frank Arthen and Laszlo J. Bollyky of American CyanamidAmerican Cyanamid
American Cyanamid was a large, diversified, American chemical manufacturer, founded by Frank Washburn in 1907. It was the only United States firm manufacturing the polio vaccine of the Sabin type....
, based on work by Edwin A. Chandross of Bell Labs
Bell Labs
Bell Laboratories is the research and development subsidiary of the French-owned Alcatel-Lucent and previously of the American Telephone & Telegraph Company , half-owned through its Western Electric manufacturing subsidiary.Bell Laboratories operates its...
in conjunction with Richard D. Sokolowski of Eh.M Labs. Other early work on chemiluminescence was carried out at the same time, by researchers under Herbert Richter at China Lake Naval Weapons Center.
There are several US patent
Patent
A patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
s for "glow stick" type devices by various inventors. Most of these are assigned to the US Navy. The earliest patent lists Bernard Dubrow, and Eugene Daniel Guth as having invented a Packaged Chemiluminescent Material in June 1965 (Patent 3,774,022). In October 1973, Clarence W. Gilliam, David Iba Sr., and Thomas N. Hall were registered as inventors of the Chemical Lighting Device (Patent 3,764,796). In June, 1974 a patent for a Chemiluminescent Device was issued with Herbert P. Richter and Ruth E. Tedrick listed as the inventors (Patent 3,819,925).
In January 1976, a patent was issued for the Chemiluminescent Signal Device with Vincent J. Esposito, Steven M. Little, and John H. Lyons listed as the inventors (Patent 3,933,118). This patent recommended a single glass ampoule that is suspended in a second substance, that when broken and mixed together provide the chemiluminescent light. The design also included a stand for the signal device so that it could be thrown from a moving vehicle and remain standing in an upright position on the road. The idea was that this would replace traditional emergency roadside flares and would be superior since it was not a fire hazard, would be easier and safer to deploy, and would not be made ineffective if struck by passing vehicles. This design with its single glass ampoule inside a plastic tube filled with a second substance that when bent breaks the glass and then is shaken to mix the substances most closely resembles the typical glow stick sold today. Many children love to wear Glow Sticks for parties and very fun events.
In December, 1977 a patent was issued for a Chemical Light Device with Richard Taylor Van Zandt as the inventor (Patent 4,064,428). This design improved upon the previous designs by adding a steel ball inside the plastic tube that when shaken would break the glass ampoule.

Practical applications
Glow-sticks are used for many purposes. They are waterproof, do not use batteries, generate negligible heat, are inexpensive, and are reasonably disposable. They can tolerate high pressures, such as those found underwater. They are used as light sources and light markers by military forcesMilitary
A military is an organization authorized by its greater society to use lethal force, usually including use of weapons, in defending its country by combating actual or perceived threats. The military may have additional functions of use to its greater society, such as advancing a political agenda e.g...
, campers, and recreational divers
Recreational diving
Recreational diving or sport diving is a type of diving that uses SCUBA equipment for the purpose of leisure and enjoyment. In some diving circles, the term "recreational diving" is used in contradistinction to "technical diving", a more demanding aspect of the sport which requires greater levels...
doing night diving
Night diving
Night diving is a type of recreational diving which takes place in darkness. The diver can experience a different underwater environment at night, because many marine animals are nocturnal....
. Glow sticks are considered the only kind of light source that is ideal safe for use immediately following an earthquake
Earthquake
An earthquake is the result of a sudden release of energy in the Earth's crust that creates seismic waves. The seismicity, seismism or seismic activity of an area refers to the frequency, type and size of earthquakes experienced over a period of time...
, hurricane, tornado
Tornado
A tornado is a violent, dangerous, rotating column of air that is in contact with both the surface of the earth and a cumulonimbus cloud or, in rare cases, the base of a cumulus cloud. They are often referred to as a twister or a cyclone, although the word cyclone is used in meteorology in a wider...
, or other catastrophic emergency situation due to the fact that they do not use any kind of electricity to work and do not create any danger of sparking. They can also be used in night-time fishing
Fishing
Fishing is the activity of trying to catch wild fish. Fish are normally caught in the wild. Techniques for catching fish include hand gathering, spearing, netting, angling and trapping....
as a lure
Fishing lure
A fishing lure is an object attached to the end of a fishing line which is designed to resemble and move like the prey of a fish. The purpose of the lure is to use movement, vibration, and colour to catch the fish's attention so it bites the hook...
.
Entertainment

Glowsticking
Glowsticking is a form of dancing with glowsticks or other glowstick-like instruments that share the same qualities: durability, consistency in light, safety to toss around, and the material of which they are made, often a soft and pliant plastic....
is the use of glow sticks in dancing. This is one of their most widely known uses in popular culture as they are frequently used for entertainment at parties (particularly rave
Rave
Rave, rave dance, and rave party are parties that originated mostly from acid house parties, which featured fast-paced electronic music and light shows. At these parties people dance and socialize to dance music played by disc jockeys and occasionally live performers...
s), concert
Concert
A concert is a live performance before an audience. The performance may be by a single musician, sometimes then called a recital, or by a musical ensemble, such as an orchestra, a choir, or a musical band...
s and dance clubs. They are carried by marching band
Marching band
Marching band is a physical activity in which a group of instrumental musicians generally perform outdoors and incorporate some type of marching with their musical performance. Instrumentation typically includes brass, woodwinds, and percussion instruments...
conductors for night-time performances; furthermore, in Hong Kong
Hong Kong
Hong Kong is one of two Special Administrative Regions of the People's Republic of China , the other being Macau. A city-state situated on China's south coast and enclosed by the Pearl River Delta and South China Sea, it is renowned for its expansive skyline and deep natural harbour...
glow sticks are widely used during the annual Mid-Autumn Festival
Mid-Autumn Festival
The Mid-Autumn Festival , also known as the Moon Festival or Mooncake Festival or Zhongqiu Festival, is a popular lunar harvest festival celebrated by Chinese and Vietnamese people. A description of the festival first appeared in Rites of Zhou, a written collection of rituals of the Western Zhou...
, and in Iceland
Iceland
Iceland , described as the Republic of Iceland, is a Nordic and European island country in the North Atlantic Ocean, on the Mid-Atlantic Ridge. Iceland also refers to the main island of the country, which contains almost all the population and almost all the land area. The country has a population...
they are commonly seen during New Year's Eve
New Year's Eve
New Year's Eve is observed annually on December 31, the final day of any given year in the Gregorian calendar. In modern societies, New Year's Eve is often celebrated at social gatherings, during which participants dance, eat, consume alcoholic beverages, and watch or light fireworks to mark the...
. Glow sticks carried by trick-or-treaters on Halloween
Halloween
Hallowe'en , also known as Halloween or All Hallows' Eve, is a yearly holiday observed around the world on October 31, the night before All Saints' Day...
neatly serve multiple functions as toys, readily visible and unusual night-time warnings to motorists, and luminous markings which enable parents to keep their brightly color-coded children in sight. Yet another aesthetic usage is for balloon-carried light effect
Balloon-carried light effect
A balloon-carried light effect is a special effect carried by a balloon, which can be fixed with a rope to the ground or free-flying.-Uses:...
s. Glow sticks are also used to create special effects in low light photography and film.
The Guinness Book of Records says the world's biggest glow stick, 8 ft 4 in tall, was built and illuminated at the opening ceremony of the second Bang Face
Bang Face
BangFace is a regular electronic dance music event that has been taking place at various venues across the UK since 2003. Starting as a monthly club night in London, it has grown to include an annual 3 day Weekender at Camber Sands, a boat party on the River Thames, as well as guest shows at...
Weekender at a holiday park in Camber Sands
Camber Sands
For historical information on the area, see Camber articleCamber Sands is a beach at the village of Camber , East Sussex, England...
, East Sussex
East Sussex
East Sussex is a county in South East England. It is bordered by the counties of Kent, Surrey and West Sussex, and to the south by the English Channel.-History:...
, England, on April 24, 2009.
A Long Island
Long Island
Long Island is an island located in the southeast part of the U.S. state of New York, just east of Manhattan. Stretching northeast into the Atlantic Ocean, Long Island contains four counties, two of which are boroughs of New York City , and two of which are mainly suburban...
haunted attraction
Haunted attraction
A haunted attraction is a form of entertainment that simulates the experience of entering a haunted location that might be inhabited by ghosts, monsters, criminals, humorous characters, and other such creatures...
called Nyctophobia utilizes glow sticks to allow their guests very limited sight in an otherwise pitch black environment.
Freezing / Heating
It is a common belief that glow sticks may be placed in a freezer to slow the chemical reaction, allowing the same sticks to be kept for two or three night's activity. In reverse, microwaving or running hot water over them speeds up the photon release and makes them brighter, but also diminishes the life of the glow stick. This, however, usually depends on the specific composition of chemicals in the particular glow stick at hand.How it works
Glow sticks give off light when two solutions are allowed to mix. The sticks consist of a small fragile container within a flexible outer container. Each container holds one of the two solutions. When the outer container is bent, it breaks the inner container, releasing the first solution into the second solution. After breaking, the tube is shaken to mix the two components. Usually to activate this reaction, you simply bend the glow stick.Glow sticks contain hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, and phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
is produced as a by-product. It is advisable to keep the mixture away from skin and to prevent accidental ingestion if the glow stick case splits or breaks. If spilled on skin the chemicals could cause slight skin irritation, swelling, or, in extreme circumstances, vomiting and nausea. Some of the chemicals used in older glow sticks were thought to potentially be carcinogen
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
s. The sensitizers used are polynuclear aromatic hydrocarbons, a class of compounds known for their carcinogenity.
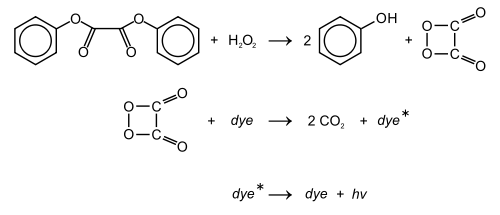
Chemistry
The glow stick contains two chemicals and a suitable fluorescent dye (sensitizer, or fluorophor). The chemicals in the glass vial are a mixture of the dye and diphenyl oxalate. The chemical inside the plastic tube is hydrogen peroxideHydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
. By mixing the peroxide with the phenyl oxalate ester, a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
takes place, yielding two molecules of phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
and one molecule of peroxyacid ester (1,2-dioxetanedione
1,2-Dioxetanedione
The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon with formula C2O4...
). The peroxyacid decomposes spontaneously
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
to carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, releasing energy that excites the dye, which then relaxes by releasing a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
. The wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of the photon—the color of the emitted light—depends on the structure of the dye.
Laboratory
A laboratory is a facility that provides controlled conditions in which scientific research, experiments, and measurement may be performed. The title of laboratory is also used for certain other facilities where the processes or equipment used are similar to those in scientific laboratories...
settings), mixing the chemicals results in a furious reaction, producing large amounts of light for only a few seconds. Heating a glow stick also causes the reaction to proceed faster and the glow stick to glow more brightly but briefly. Cooling a glow stick slows the reaction and causes it to last longer, but the light is dimmer. This can be demonstrated by refrigerating or freezing an active glow stick; when it warms up again, it will resume glowing. The dyes used in glow sticks usually exhibit fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
when exposed to ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
radiation—even a spent glow stick may therefore shine under a black light
Black light
A black light, also referred to as a UV light, ultraviolet light, or Wood's lamp, is a lamp that emits ultraviolet radiation in the long-wave range, and little visible light...
.
After activation, the glow sticks gradually shift their emission spectral distribution somewhat towards red. The light intensity is high just after activation, then exponentially decays. Leveling of this initial high output is possible by refrigerating the glow stick before activation.

Fluorophores used
- 9,10-diphenylanthracene9,10-Diphenylanthracene9,10-Diphenylanthracene is a polycyclic aromatic hydrocarbon. It has the appearance of a slightly yellow powder. 9,10-Diphenylanthracene is used as a sensitiser in chemiluminescence. In lightsticks it is used to produce blue light. It is a molecular organic semiconductor, used in blue OLEDs and...
(DPA) emits blue light - 1-chloro-9,10-diphenylanthracene (1-chloro(DPA)) and 2-chloro-9,10-diphenylanthracene2-Chloro-9,10-diphenylanthracene2-Chloro-9,10-diphenylanthracene is a fluorescent dye used in glow sticks for a blue-green glow. It is a chlorinated derivative of 9,10-diphenylanthracene.-See also:* 2-Chloro-9,10-bisanthracene...
(2-chloro(DPA)) emit blue-green light more efficiently than nonsubstituted DPA; dihydro(DPA) is purple - 9,10-bis(phenylethynyl)anthracene9,10-Bis(phenylethynyl)anthracene9,10-Bisanthracene is an aromatic hydrocarbon with the chemical formula is C30H18. It displays strong fluorescence and is used as a chemiluminescent fluorophore with high quantum efficiency....
(BPEA) emits yellow-green light with maximum at 486 nm - 1-chloro-9,10-bis(phenylethynyl)anthracene1-Chloro-9,10-bis(phenylethynyl)anthracene1-Chloro-9,10-bisanthracene is a fluorescent dye used in lightsticks. It emits yellow-green light, used in 30-minute high-intensity Cyalume sticks.-References:...
emits yellow-green light, used in 30-minute high-intensity Cyalume sticks - 2-chloro-9,10-bis(phenylethynyl)anthracene2-Chloro-9,10-bis(phenylethynyl)anthracene2-Chloro-9,10-bisanthracene is a fluorescent dye used in lightsticks. It emits green light, used in 12-hour low-intensity Cyalume sticks.-See also:*9,10-Bisanthracene*1-Chloro-9,10-bisanthracene...
emits green light, used in 12-hour low-intensity Cyalume sticks - 1,8-dichloro-9,10-bis(phenylethynyl)anthracene emits yellow light, used in Cyalume sticks
- RubreneRubreneRubrene is a red colored polycyclic aromatic hydrocarbon. Rubrene is used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks....
emits orange-yellow at 550 nm - 2,4-di-tert-butylphenyl 1,4,5,8-tetracarboxynaphthalene diamide emits deep red light, together with DPA is used to produce white or hot-pink light, depending on their ratio
- Rhodamine BRhodamine BRhodamine B is a chemical compound and a dye. It is often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers...
emits red light. Is rarely used as it breaks down in contact with phenyl oxalate, shortening the shelf life of the mixture - 5,12-bis(phenylethynyl)naphthacene5,12-Bis(phenylethynyl)naphthacene5,12-Bisnaphthacene is a fluorescent dye used in lightsticks. It yields orange light.-See also:*9,10-bisanthracene...
emits orange light - Violanthrone emits orange light at 630 nm
- 16,17-(1,2-ethylenedioxy)violanthrone emits red at 680 nm
- 16,17-dihexyloxyviolanthrone emits infraredInfraredInfrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
at 725 nm - 16,17-butyloxyviolanthrone emits infrared
- N,N'-bis(2,5,-di-tert-butylphenyl)-3,4,9,10-perylenedicarboximide emits infrared
- 1-N,N-dibutylaminoanthracene emits infrared
- 6-methylacridinium iodide emits infrared

