Julia olefination
Encyclopedia
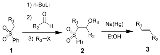
The Julia olefination is the chemical reaction
of phenyl sulfone
s (1) with aldehydes (or ketone
s) to give alkene
s (3) after alcohol
functionalization and reductive elimination using sodium amalgam
or SmI2
. The reaction is named after the French chemist Marc Julia
.
 This transformation highly favors formation of the trans-alkene.
This transformation highly favors formation of the trans-alkene.
All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is acetyl
or benzoyl
, with acetic anhydride
or benzoyl chloride
used in the preparation of 2.
Several reviews have been published.
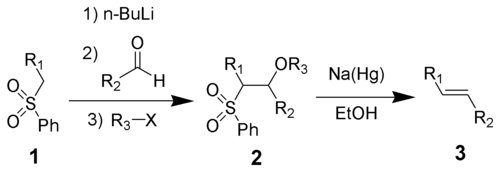
3. The alkoxide is functionalized with R3-X to give the stable intermediate 4. The exact mechanism of the sodium amalgam reduction is unknown but has been shown to proceed through a vinylic radical species (5). Protonation of the vinylic radical give the desired product (6).
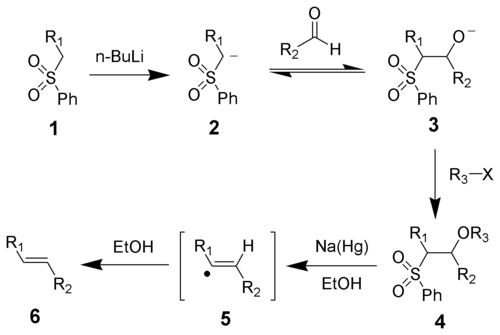 The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often.
The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often.
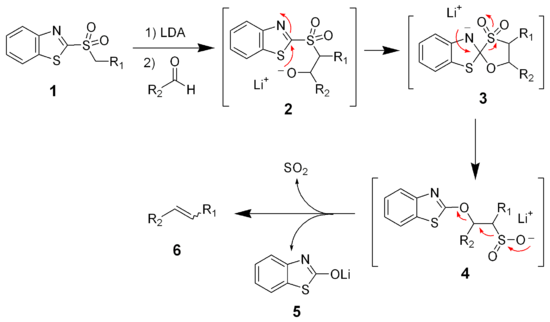
sulfone. The reaction of the benzothiazole sulfone (1) with lithium diisopropylamide
(LDA) gives a metallated benzothiazolyl sulfone, which reacts quickly with aldehydes (or ketones) to give an alkoxides intermediate (2). Unlike the phenyl sulfones, this alkoxide intermediate (2) is unstable and will undergo a Smiles rearrangement
to give the sulfinate salt 4. The sulfinate salt (4) will spontaneously eliminate sulfur dioxide
and lithium
benzothiazolone (5) producing the desired alkene (6).
 Since the benzothiazole variation of the Julia olefination does not involve equilibrating intermediates, the stereochemical outcome is a result of the stereochemistry of the initial carbonyl addition. As a result, this reaction often generates a mixture of alkene stereoisomers.
Since the benzothiazole variation of the Julia olefination does not involve equilibrating intermediates, the stereochemical outcome is a result of the stereochemistry of the initial carbonyl addition. As a result, this reaction often generates a mixture of alkene stereoisomers.
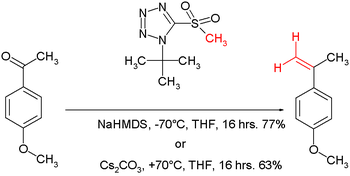
. It proceeds with the same mechanism as the benzothiazole sulfone above. In one adaptation, with t-butyltetrazoylmethyl sulfone the reaction conditions are either sodium bis(trimethylsilyl)amide
at -70°C in tetrahydrofuran
or caesium carbonate
at +70°C.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of phenyl sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s (1) with aldehydes (or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s) to give alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s (3) after alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
functionalization and reductive elimination using sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
or SmI2
Samarium(II) iodide
Samarium iodide is a green solid composed of samarium and iodine, with a melting point of 520 °C where the samarium atom has a coordination number of seven in a capped octahedral configuration...
. The reaction is named after the French chemist Marc Julia
Marc Julia
Marc Julia was a French chemist and the winner of the 1990 CNRS Gold Medal in chemistry. He discovered the Julia olefination reaction in 1973.-Biography:...
.

All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
or benzoyl
Benzoyl
In organic chemistry, benzoyl is the acyl of benzoic acid, with structure C6H5CO-. It should not be confused with benzyl, which is the radical or ion formed from the removal of one of the methyl hydrogens of toluene...
, with acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
or benzoyl chloride
Benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C6H5COCl. It is a colourless, fuming liquid with an irritating odour...
used in the preparation of 2.
Several reviews have been published.
Reaction mechanism
The initial steps are straightforward. The phenyl sulfone anion (2) reacts with an aldehyde to form the alkoxideAlkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
3. The alkoxide is functionalized with R3-X to give the stable intermediate 4. The exact mechanism of the sodium amalgam reduction is unknown but has been shown to proceed through a vinylic radical species (5). Protonation of the vinylic radical give the desired product (6).

Heteroaryl sulfones
The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway. The most popular example is the benzothiazoleBenzothiazole
Benzothiazole is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature...
sulfone. The reaction of the benzothiazole sulfone (1) with lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
(LDA) gives a metallated benzothiazolyl sulfone, which reacts quickly with aldehydes (or ketones) to give an alkoxides intermediate (2). Unlike the phenyl sulfones, this alkoxide intermediate (2) is unstable and will undergo a Smiles rearrangement
Smiles rearrangement
The Smiles rearrangement is an organic reaction and a rearrangement reaction. It is an intramolecular nucleophilic aromatic substitution of the type:...
to give the sulfinate salt 4. The sulfinate salt (4) will spontaneously eliminate sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
and lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
benzothiazolone (5) producing the desired alkene (6).

Julia-Kocienski olefination
In the Julia-Kocienski olefination the alkylating agent is a tetrazoleTetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen and one carbon atom . The simplest is tetrazole itself, CN4H2. They are unknown in nature...
. It proceeds with the same mechanism as the benzothiazole sulfone above. In one adaptation, with t-butyltetrazoylmethyl sulfone the reaction conditions are either sodium bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide
Sodium bisamide is the chemical compound with the formula 2NNa. This species, usually called NaHMDS , is a strong base used for deprotonation reactions or base catalyzed reaction...
at -70°C in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
or caesium carbonate
Caesium carbonate
Caesium carbonate is a white crystalline solid of formula Cs2CO3. It is more soluble in organic solvents than many other carbonates such as potassium carbonate, and therefore finds use as a base in organic chemistry....
at +70°C.

See also
- Horner-Wadsworth-Emmons reactionHorner-Wadsworth-Emmons reactionThe Horner-Wadsworth-Emmons reaction is the chemical reaction of stabilized phosphonate carbanions with aldehydes to produce predominantly E-alkenes....
- Johnson olefination
- Peterson olefinationPeterson olefinationThe Peterson olefination is the chemical reaction of α-silyl carbanions 1 with ketones to form a β-hydroxysilane 2 which eliminates to form alkenes 3.Several reviews have been published....
- Wittig reactionWittig reactionThe Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....

