
Passerini reaction
Encyclopedia
The Passerini reaction is a chemical reaction
involving an isocyanide
, an aldehyde
(or ketone
), and a carboxylic acid
to form a α-acyloxy amide
.
This organic reaction
was discovered by Mario Passerini in 1921 in Florence
, Italy
. It is the first isocyanide based multi-component reaction
developed, and currently plays a central role in combinatorial chemistry
.
Recently, Denmark et al. have developed an enantioselective catalyst for aymmetric Passerini reactions.
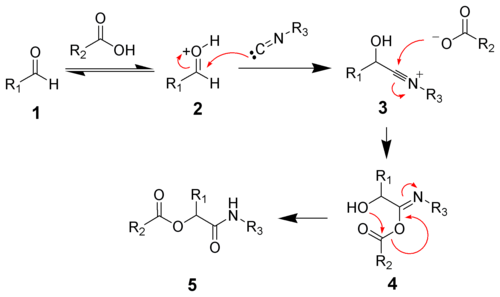
or water, the reaction proceeds by protonation of the carbonyl followed by nucleophilic addition of the isocyanide to give the nitrilium ion 3. Addition of a carboxylate gives intermediate 4. Acyl group transfer and amide tautomerization give the desired ester 5 .
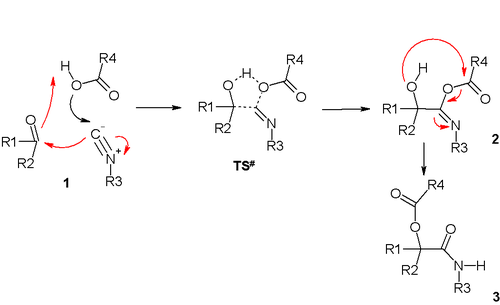 This mechanism involves a trimolecular reaction between the isocyanide
This mechanism involves a trimolecular reaction between the isocyanide
(R-NC), the carboxylic acid, and the carbonyl in a sequence of nucleophilic addition
s. The transition state
TS# is depicted as a 5-membered ring with partial covalent or double bonding. The second step of the Passerini reaction is an acyl transfer to the neighboring hydroxyl group. There is support for this reaction mechanism: the reaction proceeds in relatively non-polar solvent
s (in line with transition state) and the reaction kinetics depend on all three reactants. This reaction is a good example of a convergent synthesis
.
and forming a depsipeptide
:
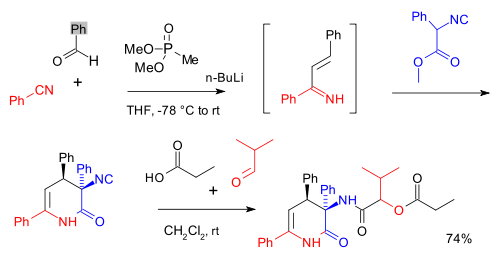 Passerini multicomponent reactions have found use in the preparation of polymers from renewable materials.
Passerini multicomponent reactions have found use in the preparation of polymers from renewable materials.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
involving an isocyanide
Isocyanide
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related cyanide , hence the prefix iso....
, an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
(or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
), and a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
to form a α-acyloxy amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
.
This organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
was discovered by Mario Passerini in 1921 in Florence
Florence
Florence is the capital city of the Italian region of Tuscany and of the province of Florence. It is the most populous city in Tuscany, with approximately 370,000 inhabitants, expanding to over 1.5 million in the metropolitan area....
, Italy
Italy
Italy , officially the Italian Republic languages]] under the European Charter for Regional or Minority Languages. In each of these, Italy's official name is as follows:;;;;;;;;), is a unitary parliamentary republic in South-Central Europe. To the north it borders France, Switzerland, Austria and...
. It is the first isocyanide based multi-component reaction
Multi-component reaction
In chemistry, a multi-component reaction , sometimes referred to as a "Multi-component Assembly Process" , is a chemical reaction where three or more compounds react to form a single product...
developed, and currently plays a central role in combinatorial chemistry
Combinatorial chemistry
Combinatorial chemistry involves the rapid synthesis or the computer simulation of a large number of different but structurally related molecules or materials...
.
Recently, Denmark et al. have developed an enantioselective catalyst for aymmetric Passerini reactions.
Ionic mechanism
In polar solvents such as methanolMethanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
or water, the reaction proceeds by protonation of the carbonyl followed by nucleophilic addition of the isocyanide to give the nitrilium ion 3. Addition of a carboxylate gives intermediate 4. Acyl group transfer and amide tautomerization give the desired ester 5 .

Concerted mechanism
In non-polar solvents and at high concentration a concerted mechanism is likely :Isocyanide
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related cyanide , hence the prefix iso....
(R-NC), the carboxylic acid, and the carbonyl in a sequence of nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
s. The transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
TS# is depicted as a 5-membered ring with partial covalent or double bonding. The second step of the Passerini reaction is an acyl transfer to the neighboring hydroxyl group. There is support for this reaction mechanism: the reaction proceeds in relatively non-polar solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s (in line with transition state) and the reaction kinetics depend on all three reactants. This reaction is a good example of a convergent synthesis
Convergent synthesis
In chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis...
.
Scope
The Passerini reactions is used in many multicomponent reactions for instance one preceded by a Horner-Wadsworth-Emmons reactionHorner-Wadsworth-Emmons reaction
The Horner-Wadsworth-Emmons reaction is the chemical reaction of stabilized phosphonate carbanions with aldehydes to produce predominantly E-alkenes....
and forming a depsipeptide
Depsipeptide
A depsipeptide is a peptide in which one or more of the amide bonds are replaced by ester bonds.Depsipeptides have often been used in research to probe the importance of hydrogen bond networks in protein folding kinetics and thermodynamics. They are also found in nature as natural products...
:


