.gif)
Glycerol (data page)
Encyclopedia
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS
Material safety data sheet
A Material Safety Data Sheet is a form with data regarding the properties of a particular substance....
) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4729 at 20°C |
| Abbe number Abbe number In physics and optics, the Abbe number, also known as the V-number or constringence of a transparent material, is a measure of the material's dispersion in relation to the refractive index... |
? |
| Dielectric constant Dielectric constant The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum... , εr |
42.5 ε0 at 25 °C |
| Bond strength Bond strength In chemistry, bond strength is measured between two atoms joined in a chemical bond. It is the degree to which each atom linked to another atom contributes to the valency of this other atom... |
? |
| Bond length Bond length - Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter... |
? |
| Bond angle | ? |
| Magnetic susceptibility Magnetic susceptibility In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field... |
? |
| Surface tension Surface tension Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface... |
63.4 mN/m at 20°C 58.6 mN/m at 90°C 51.9 mN/m at 150°C |
| Viscosity Viscosity Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity... |
4.220 Pa·s at 2.8°C 1.069 Pa·s at 20°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point Triple point In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium... |
291.8 K (18.7 °C), ? Pa |
| Critical point | 850 K (577 °C), 7500 kPa |
| Std enthalpy change of fusion, ΔfusH |
18.28 kJ/mol |
| Std entropy change of fusion Standard entropy change of fusion The entropy of fusion is the increase in entropy when melting a substance. This is always positive since the degree of disorder increases in the transition from an organized crystalline solid to the disorganized structure of a liquid... , ΔfusS |
62.7 J/(mol·K) |
| Std enthalpy change of vaporization Standard enthalpy change of vaporization The enthalpy of vaporization, , also known as the heat of vaporization or heat of evaporation, is the energy required to transform a given quantity of a substance into a gas at a given pressure .It is often measured at the normal boiling point of a substance; although tabulated values are usually... , ΔvapH |
91.7 kJ/mol |
| Std entropy change of vaporization Standard entropy change of vaporization The entropy of vaporization is the increase in entropy when vaporizing a substance. This is always positive since the degree of disorder increases in the transition from an organized crystalline solid or a slightly less organized liquid to the extremely disorganized structure of a gas... , ΔvapS |
201 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation Standard enthalpy change of formation The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states... , ΔfH |
? kJ/mol |
| Standard molar entropy Standard molar entropy In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions .... , S |
? J/(mol K) |
| Heat capacity Heat capacity Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount... , cp |
150. J/(mol K) 6°C - 11°C |
| Liquid properties | |
| Std enthalpy change of formation Standard enthalpy change of formation The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states... , ΔfH |
–669.6 kJ/mol |
| Standard molar entropy Standard molar entropy In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions .... , S |
? J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1654.3 kJ/mol |
| Heat capacity Heat capacity Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount... , cp |
221.9 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation Standard enthalpy change of formation The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states... , ΔfH |
–577.9 kJ/mol |
| Standard molar entropy Standard molar entropy In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions .... , S |
? J/(mol K) |
| Heat capacity Heat capacity Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount... , cp |
? J/(mol K) |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 |
| T in °C | 125.5 | 167.2 | 198.0 | 220.1 | 263.0 | 290.0 |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed.
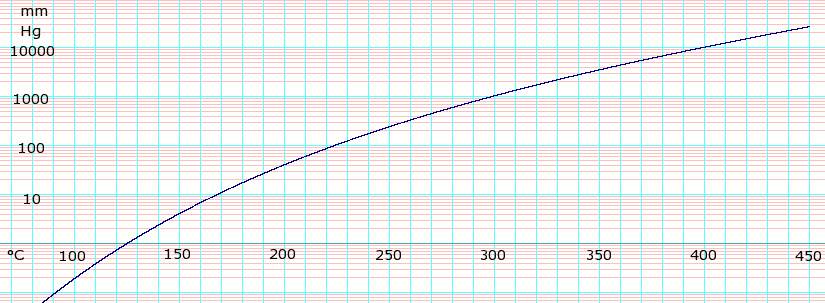
log10 of Glycerol vapor pressure. Uses formula:
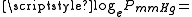
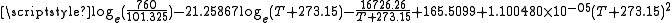 obtained from CHERIC
obtained from CHERICFreezing point of aqueous solutions
| % glycerol by volume |
10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|
| Freezing point °C |
–1.6 | –4.8 | –9.5 | –15.5 | –22.0 | –33.6 | –37.8 | –19.2 | –1.6 | 17.0 |
| Specific gravity d15° |
1.02415 | 1.04935 | 1.07560 | 1.10255 | 1.12985 | 1.15770 | 1.18540 | 1.21290 | 1.23950 | 1.26557 |
Table data obtained from Lange's Handbook of Chemistry, 10th ed. Specific gravity is at 15°C, referenced to water at 15°C.
See details on: Freezing Points of Glycerine-Water Solutions Dow Chemical
Distillation data
| Vapor-liquid Equilibrium of Glycerol/water P = 760 mmHg |
||
| BP Temp. °C |
% by mole water | |
|---|---|---|
| liquid | vapor | |
| 278.8 | 2.75 | 93.15 |
| 247.0 | 4.67 | 94.73 |
| 224.0 | 6.90 | 95.63 |
| 219.2 | 7.67 | 97.43 |
| 210.0 | 9.01 | 97.83 |
| 202.5 | 10.31 | 97.24 |
| 196.5 | 11.59 | 98.39 |
| 175.2 | 17.56 | 98.99 |
| 149.3 | 30.04 | 99.64 |
| 137.2 | 38.47 | 99.76 |
| 136.8 | 38.95 | 98.78 |
| 131.8 | 43.58 | 99.76 |
| 121.5 | 56.33 | 99.84 |
| 112.8 | 70.68 | 99.93 |
| 111.3 | 73.86 | 99.94 |
| 106.3 | 84.42 | 99.96 |
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm Nanometre A nanometre is a unit of length in the metric system, equal to one billionth of a metre. The name combines the SI prefix nano- with the parent unit name metre .The nanometre is often used to express dimensions on the atomic scale: the diameter... |
| Extinction coefficient Molar absorptivity The molar absorption coefficient, molar extinction coefficient, or molar absorptivity, is a measurement of how strongly a chemical species absorbs light at a given wavelength... , ε |
? |
| IR Infrared Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm... |
|
| Major absorption bands | ? cm−1 |
| NMR NMR spectroscopy Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained... |
|
| Proton NMR Proton NMR Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the... |
|
| Carbon-13 NMR Carbon-13 NMR Carbon-13 NMR is the application of nuclear magnetic resonance spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms... |
|
| Other NMR data | |
| MS Mass spectrometry Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and... |
|
| Masses of main fragments |
|

