
Standard molar entropy
Encyclopedia
In chemistry
, the standard molar entropy is the entropy
content of one mole
of substance, under standard conditions (not standard temperature and pressure STP
).
The standard molar entropy is usually given the symbol S°, and the units joule
s per mole kelvin
(J mol−1 K−1). Unlike standard enthalpies of formation
, the value of S° is an absolute. That is, an element in its standard state has a nonzero value of S° at room temperature. The entropy of a pure crystalline structure can be 0 J mol−1 K−1 only at 0 K, according to the third law of thermodynamics
. However, this presupposes that the material forms a 'perfect crystal' without any frozen in entropy (defects, dislocations), which is never completely true because crystals always grow at a finite temperature. This residual entropy is often quite negligible.
of substance were at 0 K, then warmed by its surroundings to 298 K, its total molar entropy would be the addition of all N individual contributions:
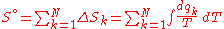
Here, dqk/T represents a very small exchange of heat energy at temperature T. The total molar entropy is the sum of many small changes in molar entropy, where each small change can be considered a reversible
process.
at STP
includes contributions from:
Changes in entropy are associated with phase transitions and chemical reactions. Chemical equations make use of the standard molar entropy of reactants and products
to find the standard entropy of reaction:
The standard entropy of reaction helps determine whether the reaction will take place spontaneously
. According to the second law of thermodynamics
, a spontaneous reaction always results in an increase in total entropy of the system and its surroundings:
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the standard molar entropy is the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
content of one mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of substance, under standard conditions (not standard temperature and pressure STP
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
).
The standard molar entropy is usually given the symbol S°, and the units joule
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s per mole kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
(J mol−1 K−1). Unlike standard enthalpies of formation
Standard enthalpy change of formation
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states...
, the value of S° is an absolute. That is, an element in its standard state has a nonzero value of S° at room temperature. The entropy of a pure crystalline structure can be 0 J mol−1 K−1 only at 0 K, according to the third law of thermodynamics
Third law of thermodynamics
The third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
. However, this presupposes that the material forms a 'perfect crystal' without any frozen in entropy (defects, dislocations), which is never completely true because crystals always grow at a finite temperature. This residual entropy is often quite negligible.
Thermodynamics
If a moleMole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of substance were at 0 K, then warmed by its surroundings to 298 K, its total molar entropy would be the addition of all N individual contributions:
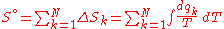
Here, dqk/T represents a very small exchange of heat energy at temperature T. The total molar entropy is the sum of many small changes in molar entropy, where each small change can be considered a reversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
process.
Chemistry
The standard molar entropy of a gasGas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
at STP
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
includes contributions from:
- The heat capacityHeat capacityHeat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
of one mole of the solid from 0 K to the melting pointMelting pointThe melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
(including heat absorbed in any changes between different crystal structureCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
s) - The latent heat of fusion of the solid.
- The heat capacity of the liquidLiquidLiquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
from the melting point to the boiling pointBoiling pointThe boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
. - The latent heat of vaporization of the liquid.
- The heat capacity of the gas from the boiling point to room temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
.
Changes in entropy are associated with phase transitions and chemical reactions. Chemical equations make use of the standard molar entropy of reactants and products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
to find the standard entropy of reaction:
- ΔS°rxn = S°products – S°reactants
The standard entropy of reaction helps determine whether the reaction will take place spontaneously
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
. According to the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
, a spontaneous reaction always results in an increase in total entropy of the system and its surroundings:
- ΔStotal = ΔSsystem + ΔSsurroundings > 0
See also
- EntropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
- HeatHeatIn physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
- Gibbs free energyGibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
- Helmholtz free energyHelmholtz free energyIn thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
- Third law of thermodynamicsThird law of thermodynamicsThe third law of thermodynamics is a statistical law of nature regarding entropy:For other materials, the residual entropy is not necessarily zero, although it is always zero for a perfect crystal in which there is only one possible ground state.-History:...
External links
- Free Energy and Chemical Reactions - Course notes for General Chemistry (R. Paselk, Humboldt State University)

