
G-factor
Encyclopedia
- For the acceleration-related quantity in mechanics, see g-forceG-forceThe g-force associated with an object is its acceleration relative to free-fall. This acceleration experienced by an object is due to the vector sum of non-gravitational forces acting on an object free to move. The accelerations that are not produced by gravity are termed proper accelerations, and...
.
A g-factor (also called g value or dimensionless magnetic moment) is a dimensionless quantity which characterizes the magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
and gyromagnetic ratio of a particle or nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. It is essentially a proportionality constant that relates the observed magnetic moment μ of a particle to the appropriate angular momentum
Angular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
quantum number
Quantum number
Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. Perhaps the most peculiar aspect of quantum mechanics is the quantization of observable quantities. This is distinguished from classical mechanics where the values can range continuously...
and the appropriate fundamental quantum unit of magnetism, usually the Bohr magneton or nuclear magneton.
Electron g-factors
There are three magnetic moments associated with an electron: One from its spin angular momentumSpin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
, one from its orbital angular momentum
Azimuthal quantum number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital...
, and one from its total angular momentum (the quantum-mechanical sum of those two components). Corresponding to these three moments are three different g-factors:
Electron spin g-factor
The most famous of these is the electron spin g-factor (more often called simply the electron g-factor), ge, defined by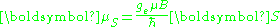
where μS is the total magnetic moment resulting from the spin of an electron, S is the magnitude of its spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
angular momentum, and μB is the Bohr magneton. In atomic physics, the electron spin g-factor is often defined as the absolute value or negative of ge:
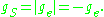
The z-component of the magnetic moment then becomes
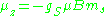
The value gS is roughly equal to 2.002319, and is known to extraordinary accuracy. The reason it is not precisely two is explained by quantum electrodynamics
Quantum electrodynamics
Quantum electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved...
calculation of the anomalous magnetic dipole moment
Anomalous magnetic dipole moment
In quantum electrodynamics, the anomalous magnetic moment of a particle is a contribution of effects of quantum mechanics, expressed by Feynman diagrams with loops, to the magnetic moment of that particle...
.
Electron orbital g-factor
Secondly, the electron orbital g-factor, gL, is defined by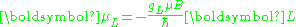
where μL is the total magnetic moment resulting from the orbital angular momentum of an electron, L is the magnitude of its orbital angular momentum, and μB is the Bohr magneton. The value of gL is exactly equal to one, by a quantum-mechanical argument analogous to the derivation of the classical magnetogyric ratio
Magnetogyric ratio
In physics, the gyromagnetic ratio of a particle or system is the ratio of its magnetic dipole moment to its angular momentum, and it is often denoted by the symbol γ, gamma...
. For an electron in an orbital with a magnetic quantum number
Magnetic quantum number
In atomic physics, the magnetic quantum number is the third of a set of quantum numbers which describe the unique quantum state of an electron and is designated by the letter m...
ml, the z-component of the orbital angular momentum is
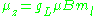
which, since gL = 1, is just μBml
Landé g-factor
Thirdly, the Landé g-factorLandé g-factor
In physics, the Landé g-factor is a particular example of a g-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921....
, gJ, is defined by
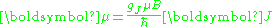
where μ is the total magnetic moment resulting from both spin and orbital angular momentum of an electron, J = L+S is its total angular momentum, and μB is the Bohr magneton. The value of gJ is related to gL and gS by a quantum-mechanical argument; see the article Landé g-factor
Landé g-factor
In physics, the Landé g-factor is a particular example of a g-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921....
.
Nucleon and nucleus g-factors
Protons, neutrons, and many nuclei have spin and magnetic moments, and therefore associated g-factors. The formula conventionally used is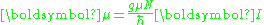
where μ is the magnetic moment resulting from the nuclear spin, I is the nuclear spin angular momentum, μN is the nuclear magneton, and g is the effective g-factor.
Muon g-factor
The muon, like the electron has a g-factor from its spin, given by the equation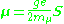
where μ is the magnetic moment resulting from the muon’s spin, S is the spin angular momentum, and mμ is the muon mass.
The fact that the muon g-factor is not quite the same as the electron g-factor is mostly explained by quantum electrodynamics and its calculation of the anomalous magnetic dipole moment
Anomalous magnetic dipole moment
In quantum electrodynamics, the anomalous magnetic moment of a particle is a contribution of effects of quantum mechanics, expressed by Feynman diagrams with loops, to the magnetic moment of that particle...
. Almost all of the small difference between the two values (99.96% of it) is due to a well-understood lack of a heavy-particle diagrams contributing to the probability for emission of a photon representing the magnetic dipole field, which are present for muons, but not electrons, in QED theory. These are entirely a result of the mass difference between the particles.
However, not all of the difference between the g-factors for electrons and muons are exactly explained by the quantum electrodynamics Standard Model
Standard Model
The Standard Model of particle physics is a theory concerning the electromagnetic, weak, and strong nuclear interactions, which mediate the dynamics of the known subatomic particles. Developed throughout the mid to late 20th century, the current formulation was finalized in the mid 1970s upon...
. The muon g-factor can, at least in theory, be affected by physics beyond
Beyond the Standard Model
Physics beyond the Standard Model refers to the theoretical developments needed to explain the deficiencies of the Standard Model, such as the origin of mass, the strong CP problem, neutrino oscillations, matter–antimatter asymmetry, and the nature of dark matter and dark energy...
the Standard Model, so it has been measured very precisely, in particular at the Brookhaven National Laboratory
Brookhaven National Laboratory
Brookhaven National Laboratory , is a United States national laboratory located in Upton, New York on Long Island, and was formally established in 1947 at the site of Camp Upton, a former U.S. Army base...
. As of November 2006, the experimentally measured value is 2.0023318416 with an uncertainty of 0.0000000013, compared to the theoretical prediction of 2.0023318361 with an uncertainty of 0.0000000010. This is a difference of 3.4 standard deviation
Standard deviation
Standard deviation is a widely used measure of variability or diversity used in statistics and probability theory. It shows how much variation or "dispersion" there is from the average...
s, suggesting beyond-the-Standard-Model physics may be having an effect.
Measured g-factor values
| Elementary Particle | g-factor | Uncertainty |
|---|---|---|
Electron 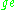 |
||
Neutron 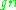 |
||
Proton 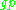 |
||
Muon 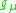 |
||
The electron g-factor is one of the most precisely measured values in physics, with its uncertainty beginning at the twelfth decimal place.
See also
- anomalous magnetic dipole momentAnomalous magnetic dipole momentIn quantum electrodynamics, the anomalous magnetic moment of a particle is a contribution of effects of quantum mechanics, expressed by Feynman diagrams with loops, to the magnetic moment of that particle...
- Electron magnetic dipole momentElectron magnetic dipole momentIn atomic physics, the electron magnetic dipole moment is the magnetic moment of an electron caused by its intrinsic property of spin.-Magnetic moment of an electron:...
- CODATA recommendations 2006

