
Landé g-factor
Encyclopedia
In physics
, the Landé g-factor is a particular example of a g-factor, namely for an electron
with both spin
and orbit
al angular momenta
. It is named after Alfred Landé
, who first described it in 1921.
In atomic physics
, it is a multiplicative term appearing in the expression for the energy levels of an atom
in a weak magnetic field
. The quantum states of electron
s in atomic orbital
s are normally degenerate in energy
, with the degenerate states all sharing the same angular momentum. When the atom is placed in a weak magnetic field, however, the degeneracy is lifted.
The factor comes about during the calculation of the first-order perturbation
in the energy of an atom when a weak uniform magnetic field (that is, weak in comparison to the system's internal magnetic field) is applied to the system. Formally we can write the factor as,
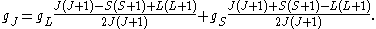
The orbital g-factor is equal to 1, and under the approximation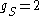 , the above expression simplifies to
, the above expression simplifies to
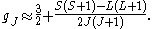
Here, J is the total electronic angular momentum, L is the orbital angular momentum, and S is the spin angular momentum. Because S=1/2 for electrons, one often sees this formula written with 3/4 in place of S(S+1). The quantities gL and gS are other g-factors of an electron.
If we wish to know the g-factor for an atom with total atomic angular momentum F=I+J,
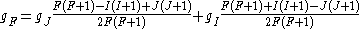
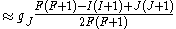
This last approximation is justified because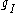 is smaller than
is smaller than 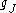 by the ratio of the electron mass to the proton mass.
by the ratio of the electron mass to the proton mass.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, the Landé g-factor is a particular example of a g-factor, namely for an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
with both spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
and orbit
Orbit
In physics, an orbit is the gravitationally curved path of an object around a point in space, for example the orbit of a planet around the center of a star system, such as the Solar System...
al angular momenta
Angular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
. It is named after Alfred Landé
Alfred Landé
Alfred Landé was a German-American physicist known for his contributions to quantum theory. He is responsible for the Landé g-factor an explanation of the Zeeman Effect.-Life and Achievements:...
, who first described it in 1921.
In atomic physics
Atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and...
, it is a multiplicative term appearing in the expression for the energy levels of an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
in a weak magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
. The quantum states of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s are normally degenerate in energy
Degenerate energy level
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
, with the degenerate states all sharing the same angular momentum. When the atom is placed in a weak magnetic field, however, the degeneracy is lifted.
The factor comes about during the calculation of the first-order perturbation
Perturbation theory (quantum mechanics)
In quantum mechanics, perturbation theory is a set of approximation schemes directly related to mathematical perturbation for describing a complicated quantum system in terms of a simpler one. The idea is to start with a simple system for which a mathematical solution is known, and add an...
in the energy of an atom when a weak uniform magnetic field (that is, weak in comparison to the system's internal magnetic field) is applied to the system. Formally we can write the factor as,
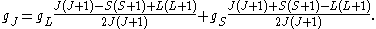
The orbital g-factor is equal to 1, and under the approximation
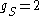 , the above expression simplifies to
, the above expression simplifies to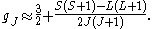
Here, J is the total electronic angular momentum, L is the orbital angular momentum, and S is the spin angular momentum. Because S=1/2 for electrons, one often sees this formula written with 3/4 in place of S(S+1). The quantities gL and gS are other g-factors of an electron.
If we wish to know the g-factor for an atom with total atomic angular momentum F=I+J,
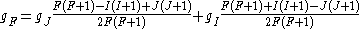
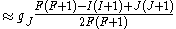
This last approximation is justified because
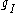 is smaller than
is smaller than 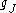 by the ratio of the electron mass to the proton mass.
by the ratio of the electron mass to the proton mass.

