
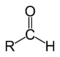
Formylation reaction
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is the catch-all name for any organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which an organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
is functionalized with a formyl group (-CH=O).
Aromatic formylation reactions via electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
include:
- DimethylformamideDimethylformamideDimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
and phosphorus oxychloride in the Vilsmeier-Haack reactionVilsmeier-Haack reactionThe Vilsmeier–Haack reaction is the chemical reaction of a substituted amide with phosphorus oxychloride and an electron-rich arene to produce an aryl aldehyde or ketone . The reaction is named after Anton Vilsmeier and Albrecht Haack... - HexamineHexamineHexamethylenetetramine is a heterocyclic organic compound with the formula 6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other chemical compounds, e.g. plastics,...
in the Duff reactionDuff reactionThe Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source...
and the Sommelet reactionSommelet reactionThe Sommelet reaction is an organic reaction in which a benzyl halide is converted to an aldehyde by action of hexamine and water..The reaction is formally an oxidation of the carbon... - Carbon monoxideCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and hydrochloric acidHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
in the Gattermann-Koch reactionGattermann-Koch reactionThe Gattermann–Koch reaction, named after the German chemists Ludwig Gattermann and Julius Arnold Koch, in organic chemistry refers to a Friedel–Crafts acylation reaction in which carbon monoxide and hydrochloric acid are used in situ with Friedel–Crafts catalyst, namely AlCl3 to produce a... - Anionic cyanides in the Gattermann aldehyde synthesis. This method synthetizes phenolic aldehydes by treatment of an aromatic compound with hydrogen chlorideHydrogen chlorideThe compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
and hydrogen cyanide (or another metallic cyanide as zinc cyanideZinc cyanideZinc cyanide is the inorganic compound with the formula Zn2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.-Structure, properties, synthesis:...
) in the presence of Lewis acid catalysts:
- ChloroformChloroformChloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
in the Reimer-Tiemann reactionReimer-Tiemann reactionThe Reimer-Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols. The reaction was discovered by Karl Ludwig Reimer and Ferdinand Tiemann... - dichloromethyl methyl etherDichloromethyl methyl etherDichloromethyl methyl ether is an organic compound that belongs to the class of ethers with a dichloromethyl group and a methyl group...
in Rieche formylationRieche formylationRieche formylation is a type of formylation reaction . The substrates are sterically hindered aromatic compounds - for example mesitylene - and the formylation reagent dichloromethyl methyl ether. The catalyst is titanium tetrachloride, the workup is acidic. The reaction is named after Alfred...
In biological systems, formylation
Formylation
Formylation is a type of posttranslational modification in which a formyl group is added to the N-terminus of a protein....
is one of the processes of posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
in which a formyl group is added to the N-terminus of a protein.

