
Duff reaction
Encyclopedia
The Duff reaction or hexamine aromatic formylation is a formylation reaction
used in organic chemistry
for the synthesis of benzaldehyde
s with hexamine
as the formyl carbon source. It is named after James Cooper Duff, who was a chemist at the College of Technology, Birmingham, roughly 1920-1950.
The electrophilic
species in this electrophilic aromatic substitution
reaction is the iminium
ion CH2+NR2. The initial reaction product is an iminium
which is hydrolyzed
to the aldehyde
. See mechanism below. The reaction requires strongly activating
substituents on the aromatic ring such as in a phenol
.
Examples are the synthesis of 3,5-di-tert-butylsalicylaldehyde
:
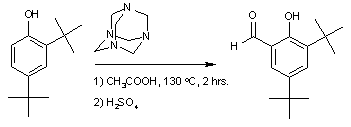 and the synthesis of syringaldehyde
and the synthesis of syringaldehyde
:
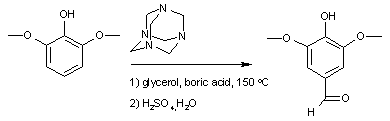
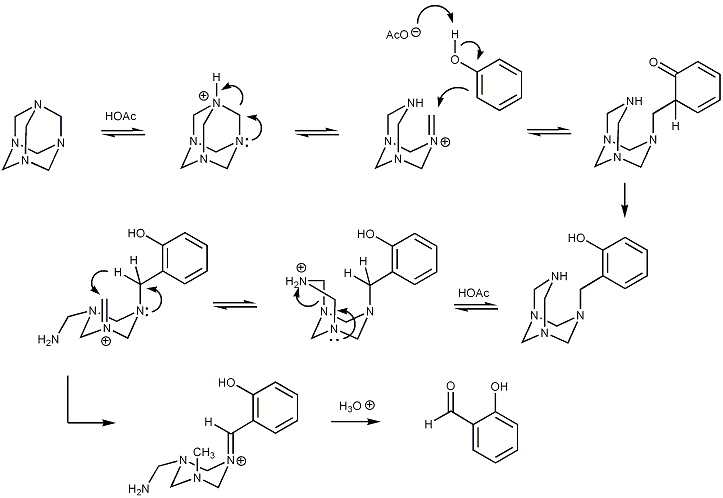
displayed below demonstrates step by step how hexamine donates a methine
group to an aromatic substrate via a series of equilibria reactions, with iminium
ion intermediates. Initially, addition to the aromatic ring results in an intermediate at the oxidation state of a benzylamine. An intramolecular redox reaction then ensues, raising the benzylic carbon to the oxidation state of an aldehyde. The oxygen atom is kindly provided by water on acid hydrolysis in the final step.
Formylation reaction
A formylation reaction in organic chemistry is the catch-all name for any organic reaction in which an organic compound is functionalized with a formyl group .Aromatic formylation reactions via electrophilic aromatic substitution include:...
used in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
for the synthesis of benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
s with hexamine
Hexamine
Hexamethylenetetramine is a heterocyclic organic compound with the formula 6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other chemical compounds, e.g. plastics,...
as the formyl carbon source. It is named after James Cooper Duff, who was a chemist at the College of Technology, Birmingham, roughly 1920-1950.
The electrophilic
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
species in this electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
reaction is the iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
ion CH2+NR2. The initial reaction product is an iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
which is hydrolyzed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
to the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
. See mechanism below. The reaction requires strongly activating
Activating group
In organic chemistry, a functional group is called an activating group if a benzene molecule to which it is attached more readily participates in electrophilic substitution reactions...
substituents on the aromatic ring such as in a phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
.
Examples are the synthesis of 3,5-di-tert-butylsalicylaldehyde
3,5-Di-tert-butylsalicylaldehyde
3,5'-Di-tert-butylsalicylaldehyde is an organic compound. It is a pale yellow solid. This aldehyde is a building block for preparing salen ligands.-Preparation:This compound is commercially available...
:
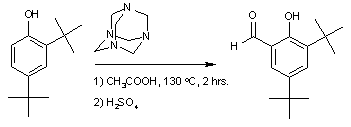
Syringaldehyde
Syringaldehyde is a naturally occurring aromatic aldehyde soluble in alcohol. Some species of insects use syringaldehyde in their chemical communication systems.Refractive index of syringaldehyde is 1.53.-Preparation:...
:
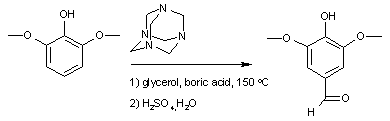
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
displayed below demonstrates step by step how hexamine donates a methine
Methine
In chemistry, methine is a trivalent functional group CH, derived formally from methane. The methine group consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen...
group to an aromatic substrate via a series of equilibria reactions, with iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
ion intermediates. Initially, addition to the aromatic ring results in an intermediate at the oxidation state of a benzylamine. An intramolecular redox reaction then ensues, raising the benzylic carbon to the oxidation state of an aldehyde. The oxygen atom is kindly provided by water on acid hydrolysis in the final step.

See also
- Bouveault aldehyde synthesisBouveault aldehyde synthesisThe Bouveault aldehyde synthesis is a one-pot chemical reaction that converts a primary alkyl halide to an aldehyde one carbon longer.-Reaction mechanism:The first step of the Bouveault aldehyde synthesis is the formation of the Grignard reagent...
- Bodroux-Chichibabin aldehyde synthesisBodroux-Chichibabin aldehyde synthesisThe Bodroux-Chichibabin aldehyde synthesis is a chemical reaction whereby a Grignard reagent is converted to an aldehyde one carbon longer.Reaction of a Grignard reagent with triethyl orthoformate gives an acetal, which can be hydrolyzed to an aldehyde. For example, the synthesis of n-hexanal:...
- Reimer-Tiemann reactionReimer-Tiemann reactionThe Reimer-Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols. The reaction was discovered by Karl Ludwig Reimer and Ferdinand Tiemann...
- Sommelet reactionSommelet reactionThe Sommelet reaction is an organic reaction in which a benzyl halide is converted to an aldehyde by action of hexamine and water..The reaction is formally an oxidation of the carbon...
- Vilsmeier-Haack reactionVilsmeier-Haack reactionThe Vilsmeier–Haack reaction is the chemical reaction of a substituted amide with phosphorus oxychloride and an electron-rich arene to produce an aryl aldehyde or ketone . The reaction is named after Anton Vilsmeier and Albrecht Haack...

