
Disgregation
Encyclopedia
In the history of thermodynamics
, disgregation was defined in 1862 by Rudolf Clausius
as the magnitude of the degree in which the molecules of a body are separated from each other. This term was modeled on certain passages in French physicist Sadi Carnot
's 1824 paper On the Motive Power of Fire that characterized the "transformations" of "working substances" (particles of a thermodynamic system
) of an engine cycle, namely "mode of aggregation", which was a precursor to the concept of entropy
, which Clausius coined in 1865. It was also a precursor to that of Ludwig Boltzmann
's 1870s theories of entropy and order and disorder
.
assumed that heat
, like a substance, cannot be diminished in quantity and that it cannot increase. Specifically, he states that in a complete engine cycle ‘that when a body has experienced any changes, and when after a certain number of transformations it returns to precisely its original state, that is, to that state considered in respect to density, to temperature, to mode of aggregation, let us suppose, I say that this body is found to contain the same quantity of heat that it contained at first, or else that the quantities of heat absorbed or set free in these different transformations are exactly compensated.’ Furthermore, he states that ‘this fact has never been called into question’ and ‘to deny this would overthrow the whole theory of heat to which it serves as a basis.’ This famous sentence, which Clausius spent fifteen years thinking about, marks the start of thermodynamics and signals the slow transition from the older caloric theory to the newer kinetic theory, in which heat is a type of energy in transit
In 1862, Clausius defined what is now known as entropy or the energetic effects related to irreversibility
as the “equivalence-values of transformations” in a thermodynamic cycle
. Clausius then signifies the difference between “reversible” (ideal) and “irreversible” (real) processes:
, as such:
Quantitatively, Clausius states the mathematical expression for this theorem is as follows. Let dQ be an element of the heat given up by the body to any reservoir of heat during its own changes, heat which it may absorb from a reservoir being here reckoned as negative, and T the absolute temperature of the body at the moment of giving up this heat, then the equation:
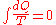
must be true for every reversible cyclical process, and the relation:
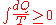
must hold good for every cyclical process which is in any way possible.
To elaborate on this, Clausius states that in all cases in which heat can perform mechanical work, these processes always admit to being reduced to the “alteration in some way or another of the arrangement of the constituent parts of the body.” To exemplify this, Clausius moves into a discussion of change of state of a body, i.e. solid, liquid, gas. For instance, he states, “when bodies are expanded by heat, their molecules being thus separated from each other: in this case the mutual attractions of the molecules on the one hand, and external opposing forces on the other, insofar as any such are in operation, have to be overcome. Again, the state of aggregation
of bodies is altered by heat, solid bodies rendered liquid, and both solid and liquid bodies being rendered aeriform: here likewise internal forces, and in general external forces also, have to be overcome.”
 Clausius then discusses the example of the melting of ice, a classic example which is used in almost all chemistry books to this day, and shows how we might represent the mechanical equivalent of work related to this energetic change mathematically:
Clausius then discusses the example of the melting of ice, a classic example which is used in almost all chemistry books to this day, and shows how we might represent the mechanical equivalent of work related to this energetic change mathematically:
is to calculate the work done in overcoming internal forces:
This description is an early formulation of the concept of entropy.
History of thermodynamics
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general...
, disgregation was defined in 1862 by Rudolf Clausius
Rudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
as the magnitude of the degree in which the molecules of a body are separated from each other. This term was modeled on certain passages in French physicist Sadi Carnot
Nicolas Léonard Sadi Carnot
Nicolas Léonard Sadi Carnot was a French military engineer who, in his 1824 Reflections on the Motive Power of Fire, gave the first successful theoretical account of heat engines, now known as the Carnot cycle, thereby laying the foundations of the second law of thermodynamics...
's 1824 paper On the Motive Power of Fire that characterized the "transformations" of "working substances" (particles of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
) of an engine cycle, namely "mode of aggregation", which was a precursor to the concept of entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, which Clausius coined in 1865. It was also a precursor to that of Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
's 1870s theories of entropy and order and disorder
Entropy (order and disorder)
In thermodynamics, entropy is commonly associated with the amount of order, disorder, and/or chaos in a thermodynamic system. This stems from Rudolf Clausius' 1862 assertion that any thermodynamic processes always "admits to being reduced to the alteration in some way or another of the arrangement...
.
Overview
In 1824, French physicist Sadi CarnotNicolas Léonard Sadi Carnot
Nicolas Léonard Sadi Carnot was a French military engineer who, in his 1824 Reflections on the Motive Power of Fire, gave the first successful theoretical account of heat engines, now known as the Carnot cycle, thereby laying the foundations of the second law of thermodynamics...
assumed that heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, like a substance, cannot be diminished in quantity and that it cannot increase. Specifically, he states that in a complete engine cycle ‘that when a body has experienced any changes, and when after a certain number of transformations it returns to precisely its original state, that is, to that state considered in respect to density, to temperature, to mode of aggregation, let us suppose, I say that this body is found to contain the same quantity of heat that it contained at first, or else that the quantities of heat absorbed or set free in these different transformations are exactly compensated.’ Furthermore, he states that ‘this fact has never been called into question’ and ‘to deny this would overthrow the whole theory of heat to which it serves as a basis.’ This famous sentence, which Clausius spent fifteen years thinking about, marks the start of thermodynamics and signals the slow transition from the older caloric theory to the newer kinetic theory, in which heat is a type of energy in transit
In 1862, Clausius defined what is now known as entropy or the energetic effects related to irreversibility
Irreversibility
In science, a process that is not reversible is called irreversible. This concept arises most frequently in thermodynamics, as applied to processes....
as the “equivalence-values of transformations” in a thermodynamic cycle
Thermodynamic cycle
A thermodynamic cycle consists of a series of thermodynamic processes transferring heat and work, while varying pressure, temperature, and other state variables, eventually returning a system to its initial state...
. Clausius then signifies the difference between “reversible” (ideal) and “irreversible” (real) processes:
Equivalence-values of transformations
He then states what he calls the “theorem respecting the equivalence-values of the transformations” or what is now known as the second law of thermodynamicsSecond law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
, as such:
Quantitatively, Clausius states the mathematical expression for this theorem is as follows. Let dQ be an element of the heat given up by the body to any reservoir of heat during its own changes, heat which it may absorb from a reservoir being here reckoned as negative, and T the absolute temperature of the body at the moment of giving up this heat, then the equation:
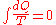
must be true for every reversible cyclical process, and the relation:
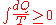
must hold good for every cyclical process which is in any way possible.
Verbal justifications
Clausius then points out the inherent difficulty in the mental comprehension of this law by stating: "although the necessity of this theorem admits of strict mathematical proof if we start from the fundamental proposition above quoted, it thereby nevertheless retains an abstract form, in which it is with difficulty embraced by the mind, and we feel compelled to seek for the precise physical cause, of which this theorem is a consequence." The justification for this law, according to Clausius, is based on the following argument:To elaborate on this, Clausius states that in all cases in which heat can perform mechanical work, these processes always admit to being reduced to the “alteration in some way or another of the arrangement of the constituent parts of the body.” To exemplify this, Clausius moves into a discussion of change of state of a body, i.e. solid, liquid, gas. For instance, he states, “when bodies are expanded by heat, their molecules being thus separated from each other: in this case the mutual attractions of the molecules on the one hand, and external opposing forces on the other, insofar as any such are in operation, have to be overcome. Again, the state of aggregation
Particle aggregation
Particle aggregation in materials science is direct mutual attraction between particles via van der Waals forces or chemical bonding....
of bodies is altered by heat, solid bodies rendered liquid, and both solid and liquid bodies being rendered aeriform: here likewise internal forces, and in general external forces also, have to be overcome.”
Definition of term
Clausius then goes on to introduce the term “disgregation”:Ice melting

Measurements of disgregation
As it is difficult to obtain direct measures of the interior forces that the molecules of the body exert on each other, Clausius states that an indirect way to obtain quantitative measures of what is now called entropyEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
is to calculate the work done in overcoming internal forces:
- In the case of the interior forces, it would accordingly be difficult—even if we did not want to measure them, but only to represent them mathematically—to find a fitting expression for them which would admit of a simple determination of the magnitude. This difficulty, however, disappears if we take into calculation, not the forces themselves, but the mechanical workMechanical workIn physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
which, in any change of arrangement, is required to overcome them. The expressions for the quantities of work are simpler than those for the corresponding forces; for the quantities of work can be all expressed, without further secondary statements, by the numbers which, having reference to the same unit, can be added together, or subtracted from one another, however various the forces may be to which they refer.
- It is therefore convenient to alter the form of the above law by introducing, instead of the forces themselves, the work done in overcoming them. In this form it reads as follows:
This description is an early formulation of the concept of entropy.

