
Electro-osmosis
Encyclopedia
Electroosmotic flow is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any other fluid conduit. Because electroosmotic velocities are independent of conduit size, as long as the double layer is much smaller than the characteristic length scale of the channel, electroosmotic flow is most significant when in small channels. Electroosmotic flow is an essential component in chemical separation techniques, notably capillary electrophoresis. Electroosmotic flow can occur in natural unfiltered water, as well as buffer
ed solutions.
by applying an electric voltage. Clay is composed of closely packed particles of sand and other minerals, and water flows through the narrow spaces between particles just as it would through a narrow glass tube. Any combination of an electrolyte
(a fluid containing dissolved ions) and insulating solid would generate electro-osmotic flow, though for water/silica the effect is particularly large. Even so, flow speeds are typically only a few millimeters per second.
in a solution. Because the chemical equilibrium between a solid surface and an electrolyte solution typically leads to the interface acquiring a net fixed electrical charge, a layer of mobile ions, known as an electrical double layer or Debye layer, forms in the region near the interface. When an electric field is applied to the fluid (usually via electrodes placed at inlets and outlets), the net charge in the electrical double layer is induced to move by the resulting Coulomb force. The resulting flow is termed electroosmotic flow.
. Unlike a parabolic profile flow generated from a pressure differential, a plug flow’s velocity profile is approximately planar, with slight variation near the electric double layer. This offers significantly less deleterious dispersive effects and can be controlled without valves, offering a high performance method for fluid separation, although many complex factors prove this control to be difficult. Because of difficulties measuring and monitoring flow in micro fluidic channels, primarily disrupting the flow pattern, most analysis is done through numerical methods and simulation.
Electroosmotic flow through micro channels can be modeled after the Navier-Stokes equation with the driving force deriving from the electric field and not the pressure differential. Thus it is governed by the continuity equation
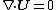
and momentum
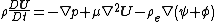
where is the velocity vector, is the density of the fluid,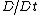 is the material derivative, is the viscosity of the fluid, is the electric charge density, is the applied electric field, and is the electric field due to the zeta potential
is the material derivative, is the viscosity of the fluid, is the electric charge density, is the applied electric field, and is the electric field due to the zeta potential
at the walls.
Laplace’s equation can describe the external electric field
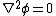
while the potential within the electric double layer is governed by
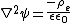
where is the dielectric constant of the electrolyte solution and is the vacuum permittivity. This equation can be further simplified using the Debye-Hückel approximation
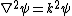
where is the Debye Length
, used to describe the characteristic thickness of the electric double layer. The equations for potential field within the double layer can be combined as:
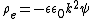
, in which electric fields are used to separate chemicals according to their electrophoretic mobility by applying an electric field to a narrow capillary, usually made of silica. In electrophoretic separations, the electroosmotic flow affects the elution time of the analytes.
It is projected that micro fluidic devices utilizing electroosmotic flow will have great application with medical research. Once controlling this flow is better understood and implemented being able to separate fluids on the atomic level will be a vital component for drug dischargers. Mixing fluids at the micro scale is currently troublesome. It is believed that electrically controlling fluids will be the method in which small fluids are mixed.
s, electro-osmosis causes proton
s moving through a proton exchange membrane
(PEM) to drag water molecules from one side (anode
) to the other (cathode
).
that differs from the cohesion-tension theory supplied in the mass flow hypothesis and others, such as cytoplasmic streaming
. Companion cells are involved in the "cyclic" withdrawal of ions ( ) from sieve tubes, and their secretion parallel to their position of withdrawal between sieve plates, resulting in polarisation of sieve plate elements alongside potential difference in pressure, and results in polar water molecules and other solutes present moved upward through the phloem.
) from sieve tubes, and their secretion parallel to their position of withdrawal between sieve plates, resulting in polarisation of sieve plate elements alongside potential difference in pressure, and results in polar water molecules and other solutes present moved upward through the phloem.
In 2003, St Petersburg University graduates applied direct electric current
to 10 mm segments of mesocotyls of maize seedlings alongside one-year linden shoots; electrolyte solutions present in the tissues moved toward the cathode that was in place, suggesting that electro-osmosis might play a role in solution transport through conductive plant tissues.
reactions to occur at the anode and cathode. This is typically electrolysis of water
, which generates hydrogen peroxide
, hydrogen ions
(acid) and hydroxide
(base) as well as oxygen
and hydrogen
gas bubbles. The hydrogen peroxide and/or pH changes generated can adversely affect biological cells and biomolecules such as proteins, while gas bubbles tend to "clog" microfluidic
systems. These problems can be alleviated by using alternative electrode materials such as conjugated polymers which can undergo the Faradaic reactions themselves, dramatically reducing electrolysis.
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
ed solutions.
History
Electroosmotic flow was first reported in 1809 by F.F. Reuss in the Proceedings of the Imperial Society of Naturalists of Moscow. He showed that water could be made to flow through a plug of clayClay
Clay is a general term including many combinations of one or more clay minerals with traces of metal oxides and organic matter. Geologic clay deposits are mostly composed of phyllosilicate minerals containing variable amounts of water trapped in the mineral structure.- Formation :Clay minerals...
by applying an electric voltage. Clay is composed of closely packed particles of sand and other minerals, and water flows through the narrow spaces between particles just as it would through a narrow glass tube. Any combination of an electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
(a fluid containing dissolved ions) and insulating solid would generate electro-osmotic flow, though for water/silica the effect is particularly large. Even so, flow speeds are typically only a few millimeters per second.
Cause
Electroosmotic flow is caused by the Coulomb force induced by an electric field on net mobile electric chargeElectric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
in a solution. Because the chemical equilibrium between a solid surface and an electrolyte solution typically leads to the interface acquiring a net fixed electrical charge, a layer of mobile ions, known as an electrical double layer or Debye layer, forms in the region near the interface. When an electric field is applied to the fluid (usually via electrodes placed at inlets and outlets), the net charge in the electrical double layer is induced to move by the resulting Coulomb force. The resulting flow is termed electroosmotic flow.
Description
The resulting flow from applying a voltage is a plug flowPlug flow
In fluid mechanics, plug flow is a simple model of the velocity profile of a fluid flowing in a pipe. In plug flow, the velocity of the fluid is assumed to be constant across any cross-section of the pipe perpendicular to the axis of the pipe...
. Unlike a parabolic profile flow generated from a pressure differential, a plug flow’s velocity profile is approximately planar, with slight variation near the electric double layer. This offers significantly less deleterious dispersive effects and can be controlled without valves, offering a high performance method for fluid separation, although many complex factors prove this control to be difficult. Because of difficulties measuring and monitoring flow in micro fluidic channels, primarily disrupting the flow pattern, most analysis is done through numerical methods and simulation.
Electroosmotic flow through micro channels can be modeled after the Navier-Stokes equation with the driving force deriving from the electric field and not the pressure differential. Thus it is governed by the continuity equation
Continuity equation
A continuity equation in physics is a differential equation that describes the transport of a conserved quantity. Since mass, energy, momentum, electric charge and other natural quantities are conserved under their respective appropriate conditions, a variety of physical phenomena may be described...
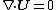
and momentum
Momentum
In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
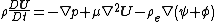
where is the velocity vector, is the density of the fluid,
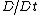 is the material derivative, is the viscosity of the fluid, is the electric charge density, is the applied electric field, and is the electric field due to the zeta potential
is the material derivative, is the viscosity of the fluid, is the electric charge density, is the applied electric field, and is the electric field due to the zeta potentialZeta potential
Zeta potential is a scientific term for electrokinetic potential in colloidal systems. In the colloidal chemistry literature, it is usually denoted using the Greek letter zeta, hence ζ-potential...
at the walls.
Laplace’s equation can describe the external electric field
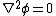
while the potential within the electric double layer is governed by
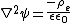
where is the dielectric constant of the electrolyte solution and is the vacuum permittivity. This equation can be further simplified using the Debye-Hückel approximation
Debye–Hückel theory
The Debye–Hückel theory was proposed by Peter Debye and Erich Hückel as a theoretical explanation for departures from ideality in solutions of electrolytes. It was based on an extremely simplified model of the electrolyte solution but nevertheless gave accurate predictions of mean activity...
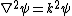
where is the Debye Length
Debye length
In plasma physics, the Debye length , named after the Dutch physicist and physical chemist Peter Debye, is the scale over which mobile charge carriers screen out electric fields in plasmas and other conductors. In other words, the Debye length is the distance over which significant charge...
, used to describe the characteristic thickness of the electric double layer. The equations for potential field within the double layer can be combined as:
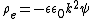
Applications
Electroosmotic flow is commonly used in microfluidic devices, soil analysis and processing, and chemical analysis, all of which routinely involve systems with highly charged surfaces, often of oxides. One example is capillary electrophoresisElectrophoresis
Electrophoresis, also called cataphoresis, is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. This electrokinetic phenomenon was observed for the first time in 1807 by Reuss , who noticed that the application of a constant electric...
, in which electric fields are used to separate chemicals according to their electrophoretic mobility by applying an electric field to a narrow capillary, usually made of silica. In electrophoretic separations, the electroosmotic flow affects the elution time of the analytes.
It is projected that micro fluidic devices utilizing electroosmotic flow will have great application with medical research. Once controlling this flow is better understood and implemented being able to separate fluids on the atomic level will be a vital component for drug dischargers. Mixing fluids at the micro scale is currently troublesome. It is believed that electrically controlling fluids will be the method in which small fluids are mixed.
Physics
In fuel cellFuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s, electro-osmosis causes proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s moving through a proton exchange membrane
Proton exchange membrane
A proton exchange membrane or polymer electrolyte membrane is a semipermeable membrane generally made from ionomers and designed to conduct protons while being impermeable to gases such as oxygen or hydrogen...
(PEM) to drag water molecules from one side (anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
) to the other (cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
).
Vascular plant biology
In vascular plant biology, electro-osmosis is also used as an alternative or supplemental explanation for the movement of polar liquids via the phloemPhloem
In vascular plants, phloem is the living tissue that carries organic nutrients , in particular, glucose, a sugar, to all parts of the plant where needed. In trees, the phloem is the innermost layer of the bark, hence the name, derived from the Greek word "bark"...
that differs from the cohesion-tension theory supplied in the mass flow hypothesis and others, such as cytoplasmic streaming
Cytoplasmic streaming
Cytoplasmic streaming is the directed flow of cytosol and organelles around large fungal and plant cells. This movement aids in the delivery of nutrients, metabolites, genetic information, and other materials to all parts of the cell...
. Companion cells are involved in the "cyclic" withdrawal of ions (
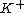 ) from sieve tubes, and their secretion parallel to their position of withdrawal between sieve plates, resulting in polarisation of sieve plate elements alongside potential difference in pressure, and results in polar water molecules and other solutes present moved upward through the phloem.
) from sieve tubes, and their secretion parallel to their position of withdrawal between sieve plates, resulting in polarisation of sieve plate elements alongside potential difference in pressure, and results in polar water molecules and other solutes present moved upward through the phloem.In 2003, St Petersburg University graduates applied direct electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
to 10 mm segments of mesocotyls of maize seedlings alongside one-year linden shoots; electrolyte solutions present in the tissues moved toward the cathode that was in place, suggesting that electro-osmosis might play a role in solution transport through conductive plant tissues.
Disadvantages
Maintaining an electric field in an electrolyte requires FaradaicFaradaic current
The faradaic current is the current generated by the reduction or oxidation of some chemical substance at an electrode. The net faradaic current is the algebraic sum of all the faradaic currents flowing through an indicator electrode or working electrode....
reactions to occur at the anode and cathode. This is typically electrolysis of water
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
, which generates hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, hydrogen ions
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
(acid) and hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
(base) as well as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas bubbles. The hydrogen peroxide and/or pH changes generated can adversely affect biological cells and biomolecules such as proteins, while gas bubbles tend to "clog" microfluidic
Microfluidics
Microfluidics deals with the behavior, precise control and manipulation of fluids that are geometrically constrained to a small, typically sub-millimeter, scale.Typically, micro means one of the following features:* small volumes...
systems. These problems can be alleviated by using alternative electrode materials such as conjugated polymers which can undergo the Faradaic reactions themselves, dramatically reducing electrolysis.
See also
- Capillary electrophoresisCapillary electrophoresisCapillary electrophoresis , also known as capillary zone electrophoresis , can be used to separate ionic species by their charge and frictional forces and hydrodynamic radius. In traditional electrophoresis, electrically charged analytes move in a conductive liquid medium under the influence of an...
- Streaming currentStreaming currentA streaming current and streaming potential are two interrelated electrokinetic phenomena studied in the areas of surface chemistry and electrochemistry...
- Streaming potential
- Zeta potentialZeta potentialZeta potential is a scientific term for electrokinetic potential in colloidal systems. In the colloidal chemistry literature, it is usually denoted using the Greek letter zeta, hence ζ-potential...
- Electroosmotic pumpElectroosmotic pumpAn electroosmotic pump , or EO pump, is used for generating flow or pressure by use of an electric field. One application of this is removing liquid flooding water from channels and gas diffusion layers and direct hydration of the proton exchange membrane in the membrane electrode assembly of the...
- electrical double layer
- microfluidicsMicrofluidicsMicrofluidics deals with the behavior, precise control and manipulation of fluids that are geometrically constrained to a small, typically sub-millimeter, scale.Typically, micro means one of the following features:* small volumes...
- electrochemistryElectrochemistryElectrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...

