
Cuprate
Encyclopedia

Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
anion. Cuprates have been known for centuries and are widely used in inorganic
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
and organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. However, interest in them has significantly increased since 1986 after the discovery of high-temperature superconductivity in a lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
by Georg Bednorz and Karl Müller
Karl Alexander Müller
Karl Alexander Müller is a Swiss physicist and Nobel laureate. He received the Nobel Prize in Physics in 1987 with Johannes Georg Bednorz for their work in superconductivity in ceramic materials.-Biography:...
.
More than 100,000 scientific papers were published on superconductivity in cuprates between 1986 and 2001,
and Bednorz and Müller were awarded the Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
only a year after their discovery.
From 1986 to 2008, almost all known high temperature superconductors were cuprate superconductors and the highest confirmed, ambient-pressure, superconducting transition temperature (Tc) was 135 K achieved in a layered cuprate HgBa2Ca2Cu3Ox in 1993.
Terminology
When a copper compound involved in a larger coordination complex has an overall negative charge, then it is referred to as a "cuprate", for example, amminepentachlorocuprate(II) [NH3CuCl5]2–. However, tetraamminedichlorocopper(II) (NH3)4CuCl2 has a neutral charge and therefore is not a cuprate ion.Inorganic cuprates
Cuprate anions form complexes with negatively charged ligands such as cyanide, hydroxide, or halides. Typical representatives of these complexes are tetracyanocuprate(I), [Cu(CN)4]3–, tetrachlorocuprate(II) [CuCl4]2– and hexahydroxocuprate(II) [Cu(OH)6]4–. There are also rare copper(III) and copper(IV) complexes such as the hexafluorocuprate(III) [CuF6]3– or hexafluorocuprate(IV) [CuF6]2–, which are strong oxidizing agentOxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
s. Whereas tetracyanocuprate(I) [Cu(CN)4]3- is colorless, most other copper(I) complexes are red-brown; copper(II) complexes have intense turquoise blue color while copper(III) and copper(IV) complexes are orange-red. Dilithium tetrachlorocuprate (Li2CuCl4) is an effective catalyst for the couplings of alkyl halides in the Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
. It is prepared by mixing lithium chloride
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
(LiCl) and copper(II) chloride
Copper(II) chloride
Copper chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper chlorides are some of the most common copper compounds, after copper sulfate....
(CuCl2) in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
.
Copper II oxide slightly dissolves in concentrated alkali to form the corresponding cuprate salts (X = alkali metal):
- 3 XOH + CuO + H2O → X3[Cu(OH)6]
Organic cuprates
Cuprates play an important role in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. The first organocopper compound, the explosive copper(I) acetylide
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
Cu2C2, was synthesized by Rudolf Christian Böttger
Rudolf Christian Böttger
Rudolf Christian Böttger was a German inorganic chemist. He conducted most of his research at the University of Frankfurt am Main...
in 1859.
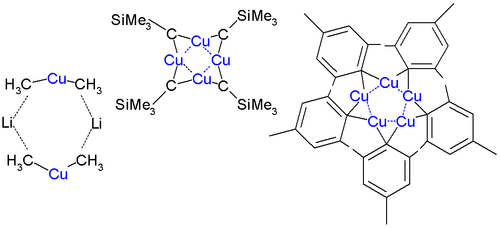
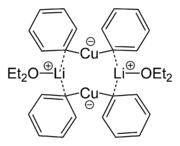
Organic cuprates usually contain an R2Cu moiety, where R is a carbon containing unit. They are rather reactive towards oxygen and water forming copper(I) oxide
Copper(I) oxide
Copper oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper. This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles, but both forms...
; they are generally insoluble in inert solvents, tend to be thermally unstable and are difficult to handle. Nevertheless, organocopper reagents are frequently used in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as alkylating reagents prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
in an inert environment. The electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of copper is much higher than its neighbor in the group 12 element
Group 12 element
A group 12 element is one of the elements in group 12 in the periodic table. This includes zinc , cadmium and mercury . The further inclusion of copernicium in group 12 is supported by recent experiments on individual Cn atoms...
s, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, suggesting less nucleophilicity
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
for carbon.
 |
 |
 |
Ionic cuprates and superconductivity
Ionic cuprate contain copper in a rare oxidation state 3+, such as CuO. Typical examples are yttrium barium copper oxideYttrium barium copper oxide
Yttrium barium copper oxide, often abbreviated YBCO, is a crystalline chemical compound with the formula YBa2Cu3O7. This material, a famous "high-temperature superconductor", achieved prominence because it was the first material to achieve superconductivity above the boiling point of liquid...
(YBCO) and bismuth strontium calcium copper oxide
Bismuth strontium calcium copper oxide
Bismuth strontium calcium copper oxide, or BSCCO , is a family of high-temperature superconductors having the generalized chemical formula Bi2Sr2Can-1CunO2n+4+x, with n=2 being the most commonly studied compound...
(BSCCO, see top figure for structure), which are the most popular high-temperature superconducting materials. Although such oxides were known for decades, only in 1986, superconductivity was discovered by Georg Bednorz and Karl Müller
Karl Alexander Müller
Karl Alexander Müller is a Swiss physicist and Nobel laureate. He received the Nobel Prize in Physics in 1987 with Johannes Georg Bednorz for their work in superconductivity in ceramic materials.-Biography:...
in a lanthanum barium copper oxide. The transition temperature in their material BaxLa5-xCu5O5(3–y) was "only" Tc = 35 K, this finding stimulated worldwide research on the synthesis and superconductivity in cuprates. Next year, the Tc value was increased to 90 K in the famous YBa2Cu3O7 (YBCO
Yttrium barium copper oxide
Yttrium barium copper oxide, often abbreviated YBCO, is a crystalline chemical compound with the formula YBa2Cu3O7. This material, a famous "high-temperature superconductor", achieved prominence because it was the first material to achieve superconductivity above the boiling point of liquid...
) cuprate. That was a breakthrough achievement because the new superconductors could be cooled by relatively cheap liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
rather than expensive liquid helium
Liquid helium
Helium exists in liquid form only at extremely low temperatures. The boiling point and critical point depend on the isotope of the helium; see the table below for values. The density of liquid helium-4 at its boiling point and 1 atmosphere is approximately 0.125 g/mL Helium-4 was first liquefied...
. Soon after, superconductivity was found in bismuth strontium calcium copper oxide
Bismuth strontium calcium copper oxide
Bismuth strontium calcium copper oxide, or BSCCO , is a family of high-temperature superconductors having the generalized chemical formula Bi2Sr2Can-1CunO2n+4+x, with n=2 being the most commonly studied compound...
(BSCCO or Bi
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
2Sr
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
2Ca
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
nCu
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
n+1O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
2n+6-d) with Tc = 95–107 K depending on the n value. Thallium barium calcium copper oxide
Thallium barium calcium copper oxide
Thallium barium calcium copper oxide, or TBCCO , is a family of high-temperature superconductors having the generalized chemical formula TlmBa2Can−1CunO2n+m+2....
(TBCCO, Tl
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
mBa
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
2Ca
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
n−1Cu
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
nO
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
2n+m+2+δ) was the next class of high-Tc cuprate superconductors with Tc = 127 K observed in
Tl2Ba2Ca2Cu3O10 (TBCCO-2223) in 1988.
In 1993, the transition temperature reached 135 K in a layered cuprate HgBa2Ca2Cu3Ox which remains the highest confirmed ambient-pressure Tc value. Whereas the low-temperature superconductivity in most conventional materials was well explained by the BCS theory
BCS theory
BCS theory — proposed by Bardeen, Cooper, and Schrieffer in 1957 — is the first microscopic theory of superconductivity since its discovery in 1911. The theory describes superconductivity as a microscopic effect caused by a "condensation" of pairs of electrons into a boson-like state...
(Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
in 1972), the mechanism of the high-temperature superconductivity in cuprates remains unsolved.
More than 100,000 scientific papers were published on superconductivity in cuprates between 1986 and 2001, and Bednorz and Müller were awarded the Nobel Prize in Physics in 1987. All superconducting cuprates are layered materials having a complex structure described as a superlattice
Superlattice
Superlattice is a periodic structure of layers of two materials. Typically, the thickness of one layer is several nanometers.- Discovery :Superlattices were discovered early in the 20th century through their special X-ray diffraction patterns....
of superconducting CuO2 layers separated by spacer layers where the misfit strain between different layers and dopants in the spacers induce a complex heterogeneity that in the superstripes
Superstripes
Superstripes are metallic heterostructures at the atomic limit where the shape resonance in the energy gap parameters ∆n is the driving mechanism for the amplification of the superconductivity critical temperature...
scenario is intrinsic for high temperature superconductivity.
BSCCO superconductors already have large-scale applications. For example, tens of kilometers of BSCCO-2223 electrical cables are being used in the Large Hadron Collider
Large Hadron Collider
The Large Hadron Collider is the world's largest and highest-energy particle accelerator. It is expected to address some of the most fundamental questions of physics, advancing the understanding of the deepest laws of nature....
– the world's largest and highest-energy particle accelerator
Particle accelerator
A particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In...
.
See also
- Benedict's reagentBenedict's reagentBenedict's reagent is a chemical reagent named after an American chemist, Stanley Rossiter Benedict....
- Corey-House synthesis
- :category:copper compounds

