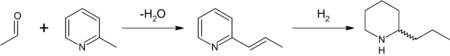Coniine
Encyclopedia
Coniine is a poisonous alkaloid
found in poison hemlock
and the yellow pitcher plant
, and contributes to hemlock's fetid smell. It is a neurotoxin
which disrupts the peripheral nervous system. It is toxic to humans and all classes of livestock; less than 0.2g is fatal to humans, with death caused by respiratory paralysis. Socrates
was put to death by way of this poison in 399 BC
. Coniine has two stereoisomers: (S)-(+)-coniine (CAS 458-88-8), which is the natural isomer present in hemlock and (R)-(-)-coniine (CAS 5985-99-9). Coniine was first synthesized by Albert Ladenburg
in 1886; it was the first of the alkaloids to be synthesized.
D-(S)-Coniine is a colourless alkaline liquid, with a penetrating odour and a burning taste; has D0° 0.8626 and D19° 0.8438, refractive index n23°D 1.4505, and is dextrorotatory, [α]19°D +15.7°. It solidifies into a soft crystalline mass at -2 °C.
Coniine is slightly soluble (1 in 90) in cold water, less so in hot water, so that a clear cold solution becomes turbid when warmed. On the other hand, the base dissolves about 25% of water at room temperature. It mixes with alcohol
in all proportions, is readily soluble in ether and most organic solvents. Coniine slowly oxidises in the air. The salts crystallise well and are soluble in water or alcohol. The hydrochloride, B•HCl, crystallises from water in rhombs, mp. 220 °C, [α]20°D +10.1°; the hydrobromide, in needles, mp. 211 °C, and the D-acid tartrate, B•C4H6O6•2 H2O, in rhombic crystals, mp. 54 °C. The platinichloride, (B•HCl)2•PtCl4•H2O, separates from concentrated solution as an oil, which solidifies to a mass of orange-yellow crystals, mp. 175 °C (dry). The aurichloride, B•HAuCl4, crystallises on standing, mp. 77 °C. The picrate
forms small yellow needles, mp. 75 °C, from hot water. The 2,4-dinitrobenzoyl- and 3,5-dinitrobenzoyl-derivates have mps. 139.0-139.5 °C and 108-9 °C respectively. Coniine dissolves in carbon disulfide
, forming a complex thiocarbamate. It gives no coloration with sulfuric
or nitric acid
. The precipitate afforded by potassium cadmium iodide solution is crystalline, mp. 118 °C, while that given by nicotine
with this reagent is amorphous. Sodium nitroprusside
gives a deep red colour, which disappears on warming, but reappears on cooling, and is changed to blue or violet by aldehyde
s.
L-(R)-Coniine has [α]21°D 15° and in other respects resembles its D-isomer, but the salts have slightly different melting points; the platinichloride has mp. 160 °C (Löffler and Friedrich report 175 °C), the aurichloride mp. 59 °C.
(α-picoline). 2-Methylpyridine was reacted with paraldehyde
in the presence of a base
to 2-propenylpyridine
in a Knoevenagel condensation
. This intermediate was reduced
with metallic sodium
in ethanol
to racemic (±) coniine (reduction by hydrogen gas is also possible). Enantiopure
coniine was obtained by chiral resolution
— fractional crystallisation
of the diastereoisomers of the salt obtained with (+)-tartaric acid
.
The initial reaction however gives a poor yield and was improved by interaction of the two reagents at 150 °C in sealed tubes to give methyl-2-picolylalkyne, which was then heated at 185 °C with hydrochloric acid
for 10 hours, producing a mixture of 2-propenylpyridine and 2-chloropropylpyridine. This mixture was reduced to rac-coniine by sodium in ethanol.
In 1907 the process was still further improved by reducing methyl-2-picolylalkine with phosphorus
and hydroiodic acid at 125 °C and treating the product with zinc
dust and water, then reducing the product with sodium in ethanol.
A number of other syntheses of coniine have been effected, of which that of Diels and Alder is of special interest. The initial adduct of pyridine
and dimethyl acetylenedicarboxylate
is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute nitric acid
is converted into trimethyl indolizine-tricarboxylate. This, on hydrolysis and decarboxylation, furnishes indolizine
, the octahydro-derivate of which, also known as octahydropyrrocoline is converted by the cyanogen bromide
method successively into the bromocyanoamide, cyanoamide and rac.-coniine. A synthesis of the alkaloid, starting from indolizine (pyrrocoline) is described by Ochiai and Tsuda.
The preparation of L-(R)-coniine by the reduction of β-coniceine (L-propenylpiperidine) by Löffler and Friedrich is interesting as a means of passing from conhydrine
to L-(R)-coniine. Hess and Eichel have shown that pelletierine is the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yields rac-coniine when its hydrazone
is heated with sodium ethoxide
in ethanol
at 156-170 °C. According to these authors, D-(S)-coniine is rendered almost optically inactive when heated with barium hydroxide
and alcohol at 180-230 °C. Leithe has shown by observation of the optical rotation of (+)-pipecolic acid
(piperidine-2-carboxylic acid) and some of its derivatives under varying conditions, that it must belong to the D-series of amino acid
s, and since (+)-conhydrine can be oxidised to (-)-pipecolic acid, and transformed through β-coniceine into L-(R)-(-)-coniine, it follows that (+)-coniine, (+)-2-methylpiperidine (α-pipecoline) and (+)-piperidine-2-carboxylic acid must all have similar spatial configurations.
causing a flaccid paralysis
. This action is similar to that of curare
. Symptoms of paralysis occur within a half hour, and death may take several hours. As the central nervous system is not affected the person remains conscious and aware until respiratory paralysis results in cessation of breathing. The muscular paralysis is an ascending flaccid paralysis as the lower limbs are affected first. The person may have an hypoxic
convulsion just prior to death but this is greatly disguised by the muscular paralysis and the person may just weakly shudder. The cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis. A poisoned person will recover if artificial ventilation (breathing) is maintained until the toxin is removed from the receptor. Historically this is the poison that killed Socrates
.
There have been a number of cases of poisoning in certain regions of Italy due to the consumption of larks and chaffinches, which eat the buds of poison hemlock
during April and May. Also, the alkaloid appears to have an addictive effect: goats, cows and pigs have all shown a preference for conium-containing foliage (up to the point of eventual death) if they survive initial exposure.
(published in 1943), also known as Murder in Retrospect, one of Agatha Christie's Hercule Poirot
mysteries.
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
found in poison hemlock
Conium
Conium is a genus of two species of highly poisonous perennial herbaceous flowering plants in the family Apiaceae, native to Europe and the Mediterranean region as Conium maculatum, and to southern Africa as Conium chaerophylloides....
and the yellow pitcher plant
Sarracenia flava
Sarracenia flava, the Yellow pitcher plant, is a carnivorous plant in the family Sarraceniaceae. Like all the Sarraceniaceae, it is native to the New World. Its range extends from southern Alabama, through Florida and Georgia, to the coastal plains of southern Virginia, North Carolina and South...
, and contributes to hemlock's fetid smell. It is a neurotoxin
Neurotoxin
A neurotoxin is a toxin that acts specifically on nerve cells , usually by interacting with membrane proteins such as ion channels. Some sources are more general, and define the effect of neurotoxins as occurring at nerve tissue...
which disrupts the peripheral nervous system. It is toxic to humans and all classes of livestock; less than 0.2g is fatal to humans, with death caused by respiratory paralysis. Socrates
Socrates
Socrates was a classical Greek Athenian philosopher. Credited as one of the founders of Western philosophy, he is an enigmatic figure known chiefly through the accounts of later classical writers, especially the writings of his students Plato and Xenophon, and the plays of his contemporary ...
was put to death by way of this poison in 399 BC
399 BC
Year 399 BC was a year of the pre-Julian Roman calendar. At the time, it was known as the Year of the Tribunate of Augurinus, Longus, Priscus, Cicurinus, Rufus and Philo...
. Coniine has two stereoisomers: (S)-(+)-coniine (CAS 458-88-8), which is the natural isomer present in hemlock and (R)-(-)-coniine (CAS 5985-99-9). Coniine was first synthesized by Albert Ladenburg
Albert Ladenburg
Albert Ladenburg was a German chemist.-Biography:Ladenburg was a member of a well known Jewish family in Mannheim. He was educated at a Realgymnasium at Mannheim and then, after the age of 15, at the technical school of Karlsruhe, where he studied mathematics and modern languages...
in 1886; it was the first of the alkaloids to be synthesized.
Isolation and properties
This alkaloid was first isolated by Giesecke, but the formula was suggested by Blyth and definitely established by Hoffmann.D-(S)-Coniine is a colourless alkaline liquid, with a penetrating odour and a burning taste; has D0° 0.8626 and D19° 0.8438, refractive index n23°D 1.4505, and is dextrorotatory, [α]19°D +15.7°. It solidifies into a soft crystalline mass at -2 °C.
Coniine is slightly soluble (1 in 90) in cold water, less so in hot water, so that a clear cold solution becomes turbid when warmed. On the other hand, the base dissolves about 25% of water at room temperature. It mixes with alcohol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
in all proportions, is readily soluble in ether and most organic solvents. Coniine slowly oxidises in the air. The salts crystallise well and are soluble in water or alcohol. The hydrochloride, B•HCl, crystallises from water in rhombs, mp. 220 °C, [α]20°D +10.1°; the hydrobromide, in needles, mp. 211 °C, and the D-acid tartrate, B•C4H6O6•2 H2O, in rhombic crystals, mp. 54 °C. The platinichloride, (B•HCl)2•PtCl4•H2O, separates from concentrated solution as an oil, which solidifies to a mass of orange-yellow crystals, mp. 175 °C (dry). The aurichloride, B•HAuCl4, crystallises on standing, mp. 77 °C. The picrate
Picrate
A picrate is a salt or an ester of picric acid . But it could also be an additional compound which picric acid forms with many aromatic hydrocarbons, aromatic amines, aliphatic amines, alkalines, and other compounds. These additional compounds are also called picrates even though they are not a...
forms small yellow needles, mp. 75 °C, from hot water. The 2,4-dinitrobenzoyl- and 3,5-dinitrobenzoyl-derivates have mps. 139.0-139.5 °C and 108-9 °C respectively. Coniine dissolves in carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
, forming a complex thiocarbamate. It gives no coloration with sulfuric
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
or nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
. The precipitate afforded by potassium cadmium iodide solution is crystalline, mp. 118 °C, while that given by nicotine
Nicotine
Nicotine is an alkaloid found in the nightshade family of plants that constitutes approximately 0.6–3.0% of the dry weight of tobacco, with biosynthesis taking place in the roots and accumulation occurring in the leaves...
with this reagent is amorphous. Sodium nitroprusside
Sodium nitroprusside
Sodium nitroprusside is the inorganic compound with the formula Na2[Fe5NO]·2H2O. This red-coloured salt, which is often abbreviated SNP, is a potent vasodilator...
gives a deep red colour, which disappears on warming, but reappears on cooling, and is changed to blue or violet by aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s.
L-(R)-Coniine has [α]21°D 15° and in other respects resembles its D-isomer, but the salts have slightly different melting points; the platinichloride has mp. 160 °C (Löffler and Friedrich report 175 °C), the aurichloride mp. 59 °C.
Synthesis
In the original synthesis of this substance by Ladenburg in 1886, he heated methylpyridinium iodide at 250 °C to obtain 2-methylpyridine2-Methylpyridine
2-Methylpyridine, or 2-picoline, is the compound described with formula C6H7N. 2-picoline is a colorless liquid that has an unpleasant odor similar to pyridine. Pyridines including 2-picoline are most crudely prepared by the reaction of acetylene and hydrogen cyanide.-Synthesis:2-Picoline was the...
(α-picoline). 2-Methylpyridine was reacted with paraldehyde
Paraldehyde
Paraldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in alcohol. Paraldehyde slowly oxidizes in air, turning brown and producing...
in the presence of a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
to 2-propenylpyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
in a Knoevenagel condensation
Knoevenagel condensation
The Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the Aldol condensation.A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule...
. This intermediate was reduced
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
with metallic sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
to racemic (±) coniine (reduction by hydrogen gas is also possible). Enantiopure
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
coniine was obtained by chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
— fractional crystallisation
Fractional crystallization (chemistry)
In chemistry, fractional crystallization is a method of refining substances based on differences in solubility. If a mixture of two or more substances in solution is allowed to crystallize, for example by allowing the temperature of the solution to decrease, the precipitate will contain more of...
of the diastereoisomers of the salt obtained with (+)-tartaric acid
Tartaric acid
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
.
The initial reaction however gives a poor yield and was improved by interaction of the two reagents at 150 °C in sealed tubes to give methyl-2-picolylalkyne, which was then heated at 185 °C with hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
for 10 hours, producing a mixture of 2-propenylpyridine and 2-chloropropylpyridine. This mixture was reduced to rac-coniine by sodium in ethanol.
In 1907 the process was still further improved by reducing methyl-2-picolylalkine with phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and hydroiodic acid at 125 °C and treating the product with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
dust and water, then reducing the product with sodium in ethanol.
A number of other syntheses of coniine have been effected, of which that of Diels and Alder is of special interest. The initial adduct of pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
and dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
is converted into trimethyl indolizine-tricarboxylate. This, on hydrolysis and decarboxylation, furnishes indolizine
Indolizine
Indolizine is a heterocyclic aromatic organic compound that is an isomer of indole. It forms the structural core of a variety of alkaloids such as swainsonine.-External links:*...
, the octahydro-derivate of which, also known as octahydropyrrocoline is converted by the cyanogen bromide
Cyanogen bromide
Cyanogen bromide is a pseudohalogen compound with the formula CNBr. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds.-Synthesis, basic properties, and structure:...
method successively into the bromocyanoamide, cyanoamide and rac.-coniine. A synthesis of the alkaloid, starting from indolizine (pyrrocoline) is described by Ochiai and Tsuda.
The preparation of L-(R)-coniine by the reduction of β-coniceine (L-propenylpiperidine) by Löffler and Friedrich is interesting as a means of passing from conhydrine
Conhydrine
Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.-Isolation and properties:This oxygenated alkaloid was isolated by Wertheim. It crystallises in colourless leaflets, has a coniine-like odour, can be sublimed, and is strongly basic. It crystallises readily from ether....
to L-(R)-coniine. Hess and Eichel have shown that pelletierine is the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yields rac-coniine when its hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
is heated with sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
in ethanol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
at 156-170 °C. According to these authors, D-(S)-coniine is rendered almost optically inactive when heated with barium hydroxide
Barium hydroxide
Barium hydroxide is the chemical compound with the formula Ba2. Also known as baryta, it is one of the principal compounds of barium. The white granular monohydrate is the usual commercial form.-Preparation:...
and alcohol at 180-230 °C. Leithe has shown by observation of the optical rotation of (+)-pipecolic acid
Pipecolic acid
Pipecolic acid is a small organic molecule which accumulates in pipecolic acidemia. It is the carboxylic acid of piperidine.It can be associated with some forms of epilepsy....
(piperidine-2-carboxylic acid) and some of its derivatives under varying conditions, that it must belong to the D-series of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, and since (+)-conhydrine can be oxidised to (-)-pipecolic acid, and transformed through β-coniceine into L-(R)-(-)-coniine, it follows that (+)-coniine, (+)-2-methylpiperidine (α-pipecoline) and (+)-piperidine-2-carboxylic acid must all have similar spatial configurations.
Pharmacology
Coniine paralyzes muscles by blocking the nicotinic receptor on the post-synaptic membrane of the neuromuscular junctionNeuromuscular junction
A neuromuscular junction is the synapse or junction of the axon terminal of a motor neuron with the motor end plate, the highly-excitable region of muscle fiber plasma membrane responsible for initiation of action potentials across the muscle's surface, ultimately causing the muscle to contract...
causing a flaccid paralysis
Flaccid paralysis
Flaccid paralysis is a clinical manifestation characterized by weakness or paralysis and reduced muscle tone without other obvious cause .-Polio:...
. This action is similar to that of curare
Curare
Curare is a common name for various arrow poisons originating from South America. The three main types of curare are:* tubocurare...
. Symptoms of paralysis occur within a half hour, and death may take several hours. As the central nervous system is not affected the person remains conscious and aware until respiratory paralysis results in cessation of breathing. The muscular paralysis is an ascending flaccid paralysis as the lower limbs are affected first. The person may have an hypoxic
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
convulsion just prior to death but this is greatly disguised by the muscular paralysis and the person may just weakly shudder. The cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis. A poisoned person will recover if artificial ventilation (breathing) is maintained until the toxin is removed from the receptor. Historically this is the poison that killed Socrates
Socrates
Socrates was a classical Greek Athenian philosopher. Credited as one of the founders of Western philosophy, he is an enigmatic figure known chiefly through the accounts of later classical writers, especially the writings of his students Plato and Xenophon, and the plays of his contemporary ...
.
There have been a number of cases of poisoning in certain regions of Italy due to the consumption of larks and chaffinches, which eat the buds of poison hemlock
Conium
Conium is a genus of two species of highly poisonous perennial herbaceous flowering plants in the family Apiaceae, native to Europe and the Mediterranean region as Conium maculatum, and to southern Africa as Conium chaerophylloides....
during April and May. Also, the alkaloid appears to have an addictive effect: goats, cows and pigs have all shown a preference for conium-containing foliage (up to the point of eventual death) if they survive initial exposure.
Coniine in Literature
Coniine is the poison used to kill Amyas Crale in Five Little PigsFive Little Pigs
Five Little Pigs is a work of detective fiction by Agatha Christie and first published in the US by Dodd, Mead and Company in May 1942 under the title of Murder in Retrospect and in UK by the Collins Crime Club in January 1943 although some sources state that publication occurred in November 1942...
(published in 1943), also known as Murder in Retrospect, one of Agatha Christie's Hercule Poirot
Hercule Poirot
Hercule Poirot is a fictional Belgian detective created by Agatha Christie. Along with Miss Marple, Poirot is one of Christie's most famous and long-lived characters, appearing in 33 novels and 51 short stories published between 1920 and 1975 and set in the same era.Poirot has been portrayed on...
mysteries.
External links
- Information on hemlock from the University of BristolUniversity of BristolThe University of Bristol is a public research university located in Bristol, United Kingdom. One of the so-called "red brick" universities, it received its Royal Charter in 1909, although its predecessor institution, University College, Bristol, had been in existence since 1876.The University is...