Citrate synthase
Encyclopedia
The enzyme citrate synthase (E.C. 2.3.3.1 [previously 4.1.3.7]) exists in nearly all living cells and stands as a pace-making enzyme in the first step of the Citric Acid Cycle
(or Krebs Cycle). Citrate synthase is localized within eukaryotic cells in the mitochondrial matrix
, but is encoded by nuclear DNA
rather than mitochondrial. It is synthesized using cytoplasmic ribosomes, then transported into the mitochondrial matrix. Citrate synthase is commonly used as a quantitative enzyme
marker for the presence of intact mitochondria.
Citrate synthase catalyzes
the condensation reaction
of the two-carbon acetate
residue from acetyl coenzyme A and a molecule of four-carbon oxaloacetate to form the six-carbon citrate
. Oxaloacetate will be regenerated after the completion of one round of the Krebs Cycle.
acetyl-CoA
+ oxaloacetate + H2O
→ citrate
+ CoA-SH
Oxaloacetate is the first substrate to bind to the enzyme. This induces the enzyme to change its conformation, and creates a binding site for the acetyl-CoA
. Only when this citroyl-CoA has formed will another conformational change cause thioester hydrolysis
and release coenzyme A. This ensures that the energy released from the thioester bond cleavage will drive the condensation.
.png)
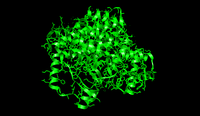 Citrate synthase's 437 amino acid residues are organized into two main subunits, each consisting of 20 alpha-helices. These alpha helices compose approximately 75% of citrate synthase's tertiary structure
Citrate synthase's 437 amino acid residues are organized into two main subunits, each consisting of 20 alpha-helices. These alpha helices compose approximately 75% of citrate synthase's tertiary structure
, while the remaining residues mainly compose irregular extensions of the structure, save a single beta-sheet of 13 residues. Between these two subunits, a single cleft exists containing the active site. Two binding sites can be found therein: one reserved for citrate or oxaloacetate and the other for Coenzyme A. The active site contains three key residues: His274, His320, and Asp375 that are highly selective in their interactions with substrates. The image to the right highlights the three key amino acids of citrate synthase's active site in its open state (the substrate is absent). The specific atoms involved in interactions are designated by color, and both a drawing and video of their mechanism can be found in the section labeled "Mechanism" below.
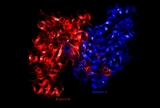
The images to the left display the tertiary structure of citrate synthase in its opened and closed form. The enzyme changes from opened to closed with the addition of one of its substrates (such as oxaloacetate).
which catalyze the conversion of acetyl-CoA
(H3CCO-SCoA) and oxaloacetate (COO-CH2COCOO-) into citrate
(COO-CH2COHCOOCH2COO-) and H-SCoA in an aldol condensation
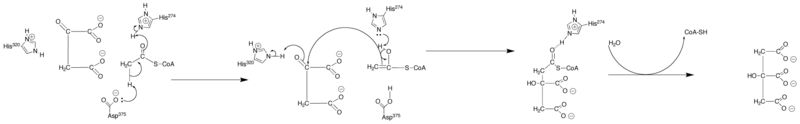
reaction. This conversion begins with the negatively charged oxygen in Asp375’s R-group deprotonating acetyl CoA’s alpha carbon. This pushes the e- to form a double-bond with the carbonyl carbon
, which in turn forces the C=O up to pick up a proton for the oxygen from one of the nitrogens in the R-group of His274. This neutralizes the R-group (by forming a lone pair
on the nitrogen) and completes the formation of an enol
intermediate (CH2COH-SCoA). At this point, His274’s amino lone pair formed in the last step attacks the proton that was added to the oxygen in the last step. The oxygen then reforms the carbonyl bond, which frees half of the C=C to initiate a nucleophilic attack to oxaloacetate’s carbonyl carbon (COO-CH2COCOO-). This frees half of the carbonyl bond to deprotonate
one of His320’s amino groups, which neutralizes one of the nitrogens in its R-group. This nucleophilic addition
results in the formation of citroyl-CoA (COOCH2CHCOOCH2COHSCoA2-). At this point, a water molecule is brought in and is deprotonated by His320’s amino group and Hydrolysis
is initiated. One of the oxygen’s lone pairs nucleophilically attacks the carbonyl
carbon of citroyl-CoA. This forms a tetrahedral intermediate and results in the ejection of –SCoA as the carbonyl reforms. The –SCoA is protonated to form HSCoA. Finally, the hydroxyl added to the carbonyl in the previous step is deprotonated and citrate (-COOCH2COHCOO-CH2COO-) is formed.
 This link connects to a video demonstrating citrate synthase's mechanism from Lehninger's Principles of Biochemistry page.
This link connects to a video demonstrating citrate synthase's mechanism from Lehninger's Principles of Biochemistry page.
:ADP
, acetyl-CoA:CoA, and NADH:NAD
, as high concentrations of ATP, acetyl-CoA, and NADH show that the energy supply is high for the cell. It is also inhibited by succinyl-CoA
and citrate
, examples of product inhibition.
The inhibition of citrate synthase by acetyl-CoA analogues has also been well documented and has been used to prove the existence of a single active site. These experiments have revealed that this single site alternates between two forms, which participate in ligase and hydrolase activity respectively.
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
(or Krebs Cycle). Citrate synthase is localized within eukaryotic cells in the mitochondrial matrix
Mitochondrial matrix
In the mitochondrion, the matrix contains soluble enzymes that catalyze the oxidation of pyruvate and other small organic molecules.The mitochondrial matrix also contains the mitochondria's DNA and ribosomes. The word "matrix" stems from the fact that this space is viscous, compared to the...
, but is encoded by nuclear DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
rather than mitochondrial. It is synthesized using cytoplasmic ribosomes, then transported into the mitochondrial matrix. Citrate synthase is commonly used as a quantitative enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
marker for the presence of intact mitochondria.
Citrate synthase catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
of the two-carbon acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
residue from acetyl coenzyme A and a molecule of four-carbon oxaloacetate to form the six-carbon citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
. Oxaloacetate will be regenerated after the completion of one round of the Krebs Cycle.
acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
+ oxaloacetate + H2O
H2O
H2O is the chemical formula for water and is also used as an abbreviation for the word "water". H2O or H2O It may also refer to:* H2O , a punk band**H2O , their self-titled debut album...
→ citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
+ CoA-SH
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
Oxaloacetate is the first substrate to bind to the enzyme. This induces the enzyme to change its conformation, and creates a binding site for the acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
. Only when this citroyl-CoA has formed will another conformational change cause thioester hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
and release coenzyme A. This ensures that the energy released from the thioester bond cleavage will drive the condensation.
Structure
.png)

Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
, while the remaining residues mainly compose irregular extensions of the structure, save a single beta-sheet of 13 residues. Between these two subunits, a single cleft exists containing the active site. Two binding sites can be found therein: one reserved for citrate or oxaloacetate and the other for Coenzyme A. The active site contains three key residues: His274, His320, and Asp375 that are highly selective in their interactions with substrates. The image to the right highlights the three key amino acids of citrate synthase's active site in its open state (the substrate is absent). The specific atoms involved in interactions are designated by color, and both a drawing and video of their mechanism can be found in the section labeled "Mechanism" below.
The images to the left display the tertiary structure of citrate synthase in its opened and closed form. The enzyme changes from opened to closed with the addition of one of its substrates (such as oxaloacetate).
Mechanism
Citrate Synthase has three key amino acids in its active siteActive site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
which catalyze the conversion of acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
(H3CCO-SCoA) and oxaloacetate (COO-CH2COCOO-) into citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
(COO-CH2COHCOOCH2COO-) and H-SCoA in an aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
reaction. This conversion begins with the negatively charged oxygen in Asp375’s R-group deprotonating acetyl CoA’s alpha carbon. This pushes the e- to form a double-bond with the carbonyl carbon
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
, which in turn forces the C=O up to pick up a proton for the oxygen from one of the nitrogens in the R-group of His274. This neutralizes the R-group (by forming a lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
on the nitrogen) and completes the formation of an enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
intermediate (CH2COH-SCoA). At this point, His274’s amino lone pair formed in the last step attacks the proton that was added to the oxygen in the last step. The oxygen then reforms the carbonyl bond, which frees half of the C=C to initiate a nucleophilic attack to oxaloacetate’s carbonyl carbon (COO-CH2COCOO-). This frees half of the carbonyl bond to deprotonate
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
one of His320’s amino groups, which neutralizes one of the nitrogens in its R-group. This nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
results in the formation of citroyl-CoA (COOCH2CHCOOCH2COHSCoA2-). At this point, a water molecule is brought in and is deprotonated by His320’s amino group and Hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
is initiated. One of the oxygen’s lone pairs nucleophilically attacks the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
carbon of citroyl-CoA. This forms a tetrahedral intermediate and results in the ejection of –SCoA as the carbonyl reforms. The –SCoA is protonated to form HSCoA. Finally, the hydroxyl added to the carbonyl in the previous step is deprotonated and citrate (-COOCH2COHCOO-CH2COO-) is formed.

Inhibition
The enzyme is inhibited by high ratios of ATPAdenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
:ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
, acetyl-CoA:CoA, and NADH:NAD
NAD
NAD may refer to:* No abnormality detected, a medical status description* No apparent distress, a status description in childbirth* NAD Electronics, a Canadian audio equipment manufacturer...
, as high concentrations of ATP, acetyl-CoA, and NADH show that the energy supply is high for the cell. It is also inhibited by succinyl-CoA
Succinyl-CoA
Succinyl-Coenzyme A, abbreviated as Succinyl-CoA or SucCoA, is a combination of succinic acid and coenzyme A.-Source:It is an important intermediate in the citric acid cycle, where it is synthesized from α-Ketoglutarate by α-ketoglutarate dehydrogenase through decarboxylation...
and citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
, examples of product inhibition.
The inhibition of citrate synthase by acetyl-CoA analogues has also been well documented and has been used to prove the existence of a single active site. These experiments have revealed that this single site alternates between two forms, which participate in ligase and hydrolase activity respectively.

