
Caesium-137
Encyclopedia
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
which is formed as a fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
by nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
.
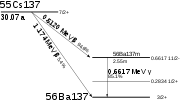
It has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of about 30.17 years, and decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
s by beta emission to a metastable nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
of barium-137: barium-137m (137mBa, Ba-137m). (About 95 percent of the nuclear decay leads to this isomer. The other 5.0 percent directly populates the ground state, which is stable.) Ba-137m has a half-life of about 153 seconds, and it is responsible for all of the emissions of gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s. One gram of caesium-137 has an activity of 3.215 terabecquerel
Becquerel
The becquerel is the SI-derived unit of radioactivity. One Bq is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. The Bq unit is therefore equivalent to an inverse second, s−1...
(TBq).
The photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
energy of Ba-137m is 662 keV. These photons can be useful in food irradiation
Food irradiation
Food irradiation is the process of exposing food to ionizing radiation to destroy microorganisms, bacteria, viruses, or insects that might be present in the food. Further applications include sprout inhibition, delay of ripening, increase of juice yield, and improvement of re-hydration...
and in the radiotherapy of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
. Caesium-137 is not widely-used for industrial radiography
Radiography
Radiography is the use of X-rays to view a non-uniformly composed material such as the human body. By using the physical properties of the ray an image can be developed which displays areas of different density and composition....
because it is quite chemically reactive, and hence, difficult to handle. Also the salts of caesium are very soluble in water, and this complicates the safe handling of caesium. Cobalt-60
Cobalt-60
Cobalt-60, , is a synthetic radioactive isotope of cobalt. Due to its half-life of 5.27 years, is not found in nature. It is produced artificially by neutron activation of . decays by beta decay to the stable isotope nickel-60...
, , is preferred for radiography, since it is chemically a rather nonreactive metal offering higher energy gamma-ray photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s. Caesium-137 can be found in some moisture and density gauges, flow meters, and related sensors.
Uses
Caesium-137 has a small number of practical uses. In small amounts, it is used to calibrate radiation-detection equipment. It is used as a gamma emitter for oilfield wireline density measurements. It is also sometimes used in cancer treatment, and it is also used industrially in gauges for measuring liquid flows and the thickness of materials.Radioactive caesium in the environment
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
. As of 2005, caesium-137 is the principal source of radiation in the zone of alienation
Zone of alienation
The Chernobyl Nuclear Power Plant Zone, which is sometimes referred to as The Chernobyl Zone, The 30 Kilometer Zone, The Zone of Alienation, or simply The Zone The Chernobyl Nuclear Power Plant Zone, which is sometimes referred to as The Chernobyl Zone, The 30 Kilometer Zone, The Zone of...
around the Chernobyl nuclear power plant
Chernobyl Nuclear Power Plant
The Chernobyl Nuclear Power Plant or Chornobyl Nuclear Power Plant is a decommissioned nuclear power station near the city of Pripyat, Ukraine, northwest of the city of Chernobyl, from the Ukraine–Belarus border, and about north of Kiev. Reactor 4 was the site of the Chernobyl disaster in...
. Together with caesium-134, iodine-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
, and strontium-90
Strontium-90
Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard...
, caesium-137 was among the isotopes, distributed by the reactor explosion, which constitute the greatest risk to health.
As of April 2011, it was also being found in the plumes emanating from the continuing leakage at the Fukushima reactors in Japan. In July 2011, meat from 11 cows shipped to Tokyo from Fukushima prefecture was found to have 3 to 6 times the legal limit of 500 becquerels per kilogram of radioactive caesium.
The mean contamination of caesium-137 in Germany following the Chernobyl disaster was 2000 to 4000 Bq/m2. This corresponds to a contamination of 1 mg/km2 of caesium-137, totaling about 500 grams deposited over all of Germany.
All caesium-137 existing today is unique in that it is totally anthropogenic (man-made). Unlike most other radioisotopes,
caesium-137 is not produced from its non-radioactive isotope but from uranium, meaning that until now, it has not occurred on Earth for billions of years
Natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where analysis of isotope ratios has shown that self-sustaining nuclear chain reactions have occurred. The existence of this phenomenon was discovered in 1972 at Oklo in Gabon, Africa, by French physicist Francis Perrin. The conditions under...
. By observing the characteristic gamma rays emitted by this isotope, it is possible to determine whether the contents of a given sealed container were made before or after the advent of atomic bomb explosions. This procedure has been used by researchers to check the authenticity of certain rare wines, most notably the purported "Jefferson bottles".
Health risk of radioactive caesium
Caesium-137 reacts with water producing a water-soluble compound (caesium hydroxideCaesium hydroxide
Caesium hydroxide is a chemical compound consisting of an atom of caesium and a hydroxide group . It is a powerful base, much like other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide...
), and the biological behavior of caesium is similar to that of potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
and rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
. After entering the body, caesium gets more or less uniformly distributed throughout the body, with higher concentration in muscle tissues and lower in bones. The biological half-life
Biological half-life
The biological half-life or elimination half-life of a substance is the time it takes for a substance to lose half of its pharmacologic, physiologic, or radiologic activity, as per the MeSH definition...
of caesium is rather short at about 70 days. Experiments with dogs showed that a single dose of 3800 μCi
Curie
The curie is a unit of radioactivity, defined asThis is roughly the activity of 1 gram of the radium isotope 226Ra, a substance studied by the pioneers of radiology, Marie and Pierre Curie, for whom the unit was named. In addition to the curie, activity can be measured using an SI derived unit,...
/kg (140 MBq/kg, or approximately 44 μg/kg) is lethal within three weeks.
Accidental ingestion of caesium-137 can be treated with Prussian blue
Prussian blue
Prussian blue is a dark blue pigment with the idealized formula Fe718. Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents....
, which binds to it chemically and then speeds its expulsion from the body.
Incidents
The improper handling of caesium-137 gamma rayGamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
sources can lead to release of this radio-isotope and radiation injuries. Perhaps the best-known case is the Goiânia accident
Goiânia accident
The Goiânia accident was a radioactive contamination accident that occurred on September 13, 1987, at Goiânia, in the Brazilian State of Goiás after an old radiotherapy source was taken from an abandoned hospital site in the city...
of 1985, in which an improperly-disposed-of radiation therapy system from an abandoned clinic in the city of Goiânia
Goiânia
-Climate:The city has a tropical wet and dry climate with an average temperature of . There's a wet season, from October to April, and a dry one, from May to September. Annual rainfall is around 1,520 mm....
, Brazil
Brazil
Brazil , officially the Federative Republic of Brazil , is the largest country in South America. It is the world's fifth largest country, both by geographical area and by population with over 192 million people...
, was scavenged from a junkyard, and the glowing caesium salt sold to curious, uneducated buyers. This led to four deaths and serious injuries from radiation exposure.
Caesium gamma-ray sources that have been encased in metallic housings can be mixed-in with scrap metal on its way to smelters, resulting in production of steel contaminated with radioactivity.
One notable example was the Acerinox accident
Acerinox accident
The Acerinox accident was an incident of radioactive contamination in Southern Spain. In May 1998, a caesium-137 source managed to pass through the monitoring equipment in a Acerinox scrap metal reprocessing plant in Los Barrios, Spain. When melted, the caesium-137 caused the release of a...
of 1998, when the Spanish
Spain
Spain , officially the Kingdom of Spain languages]] under the European Charter for Regional or Minority Languages. In each of these, Spain's official name is as follows:;;;;;;), is a country and member state of the European Union located in southwestern Europe on the Iberian Peninsula...
recycling company Acerinox
Acerinox
Acerinox, S.A. is a stainless steel manufacturing conglomerate group based in Spain. The company was founded in 1970, and initially received technical support from the Japanese firm Nisshin Steel. Nisshin continues to hold approximately 15% of Acerinox as of April 2010. The headquarters are in...
accidentally melted down a mass of radioactive caesium-137 that came from a gamma-ray generator.
In 2009, a Chinese cement company (in Tongchuan, Shaanxi Province) was demolishing an old, unused cement plant and did not follow standards for handling radioactive materials. This caused some caesium-137 from a measuring instrument to be included with eight truckloads of scrap metal
Scrap Metal
Scrap Metal were a band from Broome, Western Australia who played rock music with elements of country and reggae. The members had Aboriginal, Irish, Filipino, French, Chinese, Scottish, Indonesian and Japanese heritage. The band toured nationally as part of the Bran Nue Dae musical and with...
on its way to a steel mill
Steel mill
A steel mill or steelworks is an industrial plant for the manufacture of steel.Steel is an alloy of iron and carbon. It is produced in a two-stage process. First, iron ore is reduced or smelted with coke and limestone in a blast furnace, producing molten iron which is either cast into pig iron or...
, where the radioactive caesium was melted down into the steel.

