
Natural nuclear fission reactor
Encyclopedia
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
deposit where analysis of isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
ratio
Ratio
In mathematics, a ratio is a relationship between two numbers of the same kind , usually expressed as "a to b" or a:b, sometimes expressed arithmetically as a dimensionless quotient of the two which explicitly indicates how many times the first number contains the second In mathematics, a ratio is...
s has shown that self-sustaining nuclear chain reaction
Nuclear chain reaction
A nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The specific nuclear reaction may be the fission of heavy isotopes or the fusion of light isotopes...
s have occurred. The existence of this phenomenon was discovered in 1972 at Oklo
Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African state of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.-History:...
in Gabon
Gabon
Gabon , officially the Gabonese Republic is a state in west central Africa sharing borders with Equatorial Guinea to the northwest, Cameroon to the north, and with the Republic of the Congo curving around the east and south. The Gulf of Guinea, an arm of the Atlantic Ocean is to the west...
, Africa, by French physicist
Physicist
A physicist is a scientist who studies or practices physics. Physicists study a wide range of physical phenomena in many branches of physics spanning all length scales: from sub-atomic particles of which all ordinary matter is made to the behavior of the material Universe as a whole...
Francis Perrin
Francis Perrin
Francis Perrin was a French physicist,the son of Nobel prize-winning physicist Jean Perrin.- Physicist :Francis Perrin was born in Paris and attended École Normale Supérieure in Paris.In 1928 he obtained a doctorate in mathematical sciences from the faculté des sciences of Paris, based upon a...
. The conditions under which a natural nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
could exist had been predicted in 1956 by Paul Kazuo Kuroda. The conditions found were very similar to what was predicted.
Oklo is the only known location for this in the world and consists of 16 sites at which self-sustaining nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
reactions took place approximately 1.7 billion years ago, and ran for a few hundred thousand years, averaging 100 kW of power output during that time.
History
In May 1972 at the PierrelattePierrelatte
Pierrelatte is a commune in the Drôme department in southeastern France.-Population:-References:*...
uranium enrichment
Enriched uranium
Enriched uranium is a kind of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Natural uranium is 99.284% 238U isotope, with 235U only constituting about 0.711% of its weight...
facility in France, routine mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
comparing UF6
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
samples from the Oklo Mine
Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African state of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.-History:...
, located in Gabon
Gabon
Gabon , officially the Gabonese Republic is a state in west central Africa sharing borders with Equatorial Guinea to the northwest, Cameroon to the north, and with the Republic of the Congo curving around the east and south. The Gulf of Guinea, an arm of the Atlantic Ocean is to the west...
, Central Africa
Central Africa
Central Africa is a core region of the African continent which includes Burundi, the Central African Republic, Chad, the Democratic Republic of the Congo, and Rwanda....
, showed a discrepancy in the amount of the isotope. Normally the concentration is 0.720%; these samples had only 0.717% – a significant difference. This discrepancy required explanation, as all uranium handling facilities must meticulously account for all fissionable isotopes to assure that none are diverted for weapons
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
purposes. Thus the French Commissariat à l'énergie atomique (CEA)
Commissariat à l'Énergie Atomique
The Commissariat à l'énergie atomique et aux énergies alternatives or CEA, is a French “public establishment related to industrial and commercial activities” whose mission is to develop all applications of nuclear power, both civilian and military...
began an investigation. A series of measurements of the relative abundances of the two most significant isotopes of the uranium mined at Oklo showed anomalous results compared to those obtained for uranium from other mines. Further investigations into this uranium deposit discovered uranium ore with a to ratio as low as 0.440%. Subsequent examination of other isotopes showed similar anomalies, such as Nd
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
and Ru
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
as described in more detail below.
This loss in is exactly what happens in a nuclear reactor. A possible explanation therefore was that the uranium ore had operated as a natural fission reactor. Other observations led to the same conclusion, and on September 25, 1972, the CEA announced their finding that self-sustaining nuclear chain reactions had occurred on Earth about 2 billion years ago. Later, other natural nuclear fission reactors were discovered in the region.
Nd
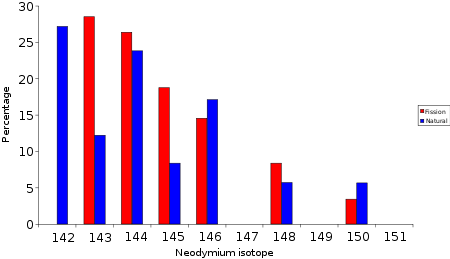
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
and other elements were found with isotopic compositions different from what is customarily found on Earth. For example, natural neodymium contains 27% ; the Nd at Oklo contained less than 6% but contained more . Subtracting the natural isotopic Nd abundance from the Oklo-Nd, the isotopic composition matched that produced by the fissioning of .
Ru
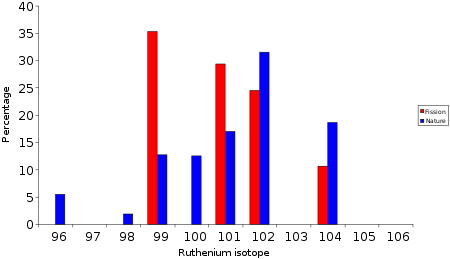
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
at Oklo found a much higher concentration than expected (27-30% vs. 12.7%). This anomaly could be explained by the decay of to . In the bar chart below the normal natural isotope signature of ruthenium is compared with that for fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
ruthenium which is the result of the fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
of with thermal neutrons. It is clear that the fission ruthenium has a different isotope signature. The level of in the fission product mixture is low because of a long lived (half life = 1019 years) isotope of molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
. On the time scale of when the reactors were in operation very little decay to will have occurred.
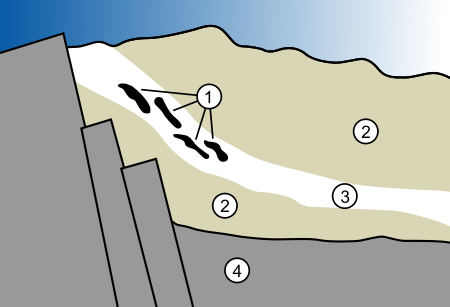
Mechanism of the reactors
The natural nuclear reactor formed when a uranium-rich mineral deposit became inundated with groundwaterGroundwater
Groundwater is water located beneath the ground surface in soil pore spaces and in the fractures of rock formations. A unit of rock or an unconsolidated deposit is called an aquifer when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock...
that acted as a neutron moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
, and a nuclear chain reaction
Nuclear chain reaction
A nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The specific nuclear reaction may be the fission of heavy isotopes or the fusion of light isotopes...
took place. The heat generated from the nuclear fission caused the groundwater to boil away, which slowed or stopped the reaction. After cooling of the mineral deposit, the water returned and the reaction started again. These fission reactions were sustained for hundreds of thousands of years, until a chain reaction could no longer be supported.
Fission of uranium normally produces five known isotopes of the fission-product gas xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
; all five have been found trapped in the remnants of the natural reactor, in varying concentrations. The concentrations of xenon isotopes, found trapped in mineral formations 2 billion years later, make it possible to calculate the specific time intervals of reactor operation: approximately 30 minutes of criticality followed by 2 hours and 30 minutes of cooling down to complete a 3-hour cycle.
A key factor that made the reaction possible was that, at the time the reactor went critical
Critical mass
A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The...
1.7 billion years ago, the fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
isotope made up about 3.1% of the natural uranium, which is comparable to the amount used in some of today's reactors. (The remaining 97% was non-fissile .) Because has a shorter half life than , and thus decays more rapidly, the current abundance of in natural uranium is about 0.7%. A natural nuclear reactor is therefore no longer possible on Earth without heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
or graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
.
The Oklo uranium ore deposits are the only known sites in which natural nuclear reactors existed. Other rich uranium ore bodies would also have had sufficient uranium to support nuclear reactions at that time, but the combination of uranium, water and physical conditions needed to support the chain reaction was unique to the Oklo ore bodies.
Another factor which probably contributed to the start of the Oklo natural nuclear reactor at 2 billion years, rather than earlier, was the increasing oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
content in the Earth's atmosphere. Uranium is naturally present in the rocks of the earth, and the abundance of fissile was at least 3% or higher at all times prior to reactor startup. Uranium is soluble in water only in the presence of oxygen. Therefore, the rising oxygen levels during the aging of earth may have allowed uranium to be dissolved and transported with groundwater to places where a high enough concentration could accumulate to form rich uranium ore bodies. Without the new aerobic environment available on earth at the time, these concentrations probably could not have taken place.
It is estimated that nuclear reactions in the uranium in centimeter- to meter-sized veins consumed about five tons of and elevated temperatures to a few hundred degrees Celsius. Most of the non-volatile fission products and actinides have only moved centimeters in the veins during the last 2 billion years. This offers a case study of how radioactive isotopes migrate through the Earth's crust.
Relation to the atomic fine-structure constant
The natural reactor of Oklo has been used to check if the atomic fine-structure constantFine-structure constant
In physics, the fine-structure constant is a fundamental physical constant, namely the coupling constant characterizing the strength of the electromagnetic interaction. Being a dimensionless quantity, it has constant numerical value in all systems of units...
 might have changed over the past 2 billion years. That is because
might have changed over the past 2 billion years. That is because  influences the rate of various nuclear reactions. For example, captures a neutron to become , and since the rate of neutron capture depends on the value of
influences the rate of various nuclear reactions. For example, captures a neutron to become , and since the rate of neutron capture depends on the value of  , the ratio of the two samarium isotopes in samples from Oklo can be used to calculate the value of
, the ratio of the two samarium isotopes in samples from Oklo can be used to calculate the value of  from 2 billion years ago.
from 2 billion years ago.Several studies have analysed the relative concentrations of radioactive isotopes left behind at Oklo, and most (but not all) have concluded that nuclear reactions then were much the same as they are today, which implies
 was the same too.
was the same too.
