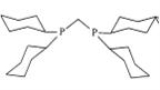
Bite angle
Overview
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s in inorganic and organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
. Together with ligand cone angle
Ligand cone angle
The ligand cone angle is a measure of the size of a ligand. It is defined as the solid angle formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone . Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any...
, this parameter is relevant to diphosphine ligands, which are used in industrial processes such as hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
and hydrocyanation
Hydrocyanation
Hydrocyanation is, most fundamentally, the process whereby H+ and –CN ions are added to a molecular substrate. Usually the substrate is an alkene and the product is a nitrile. When –CN is a ligand in a transition metal complex, its basicity makes it difficult to dislodge, so, in this...
. Even subtle changes in these parameters can significantly influence the selectivity and rate of catalytic reactions.
One of the first applications of phosphine ligands in catalysis was the use of triphenylphosphine in “Reppe
Walter Reppe
Walter Julius Reppe was a German chemist. He is notable for his contributions to the chemistry of acetylene.-Education and career:...
” chemistry (1948), which included reactions of alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s, carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, and alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s.
Unanswered Questions

