Hydrocyanation
Encyclopedia
Hydrocyanation is, most fundamentally, the process whereby H+
and –CN
ion
s are added to a molecular
substrate
. Usually the substrate is an alkene
and the product is a nitrile
. When –CN is a ligand
in a transition metal complex, its basicity makes it difficult to dislodge, so, in this respect, hydrocyanation is remarkable. Since cyanide is both a good σ–donor and π–acceptor its presence accelerates the rate
of substitution
of ligands trans from itself, the trans effect
.1 A key step in hydrocyanation is the oxidative addition
of hydrogen cyanide to low–valent metal complexes. In hydrocyanation of unsaturated carbonyls addition over the alkene competes with addition over the carbonyl group.
s and alkyne
s with copper
, palladium
, and most commonly, nickel
catalysts. Industrial hydrocyanation utilizes phosphite
(P(OR)3) complexes of nickel. Phosphites give excellent catalysts, whereas the related phosphine
(PR3) ligands, which are more basic, are catalytically inactive. Chiral
, chelating aryl
diphosphite complexes are commonly employed in asymmetric
hydrocyanation. An example of a nickel–phosphite catalyzed hydrocyanation of ethene.
Lewis acid
s, such as B(C6H5)3, can increase hydrocyanation rates and allow for lower operating temperature
s. Triphenylboron may derive this ability from sterically protecting the –CN as it is bound to nitrogen
. Rates can also be amplified with electron–withdrawing groups (NO2, CF3, CN, C(=O)OR, C(=O)R) on the phosphite ligands, because they stabilize Ni(0)
.
A major problem when using nickel catalysts for hydrocyanation is the production of Ni0(CN)x as a result of excess HCN.3 Bulky ligands impede the formation of these unreactive Ni0(CN)x complexes.
s, amine
s, carboxylic acid
s, and ester compounds.
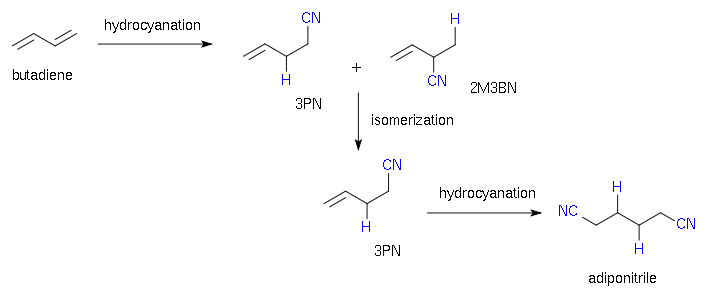
The most popular industrial usage of nickel-catalyzed hydrocyanation is for adiponitrile
(NC–(CH2)4–CN) synthesis from 1,3–butadiene (CH2=CH–CH=CH2). Adiponitrile is a precursor
to hexamethylenediamine
(H2N–(CH2)6–NH2), which is used for the production of certain kinds of Nylon
. The DuPont
ADN process to give adiponitrile is shown below:
This process consists of three steps: hydrocyanation of butadiene to a mixture of 2-methyl-3-butenenitrile (2M3BM) and 3-pentenenitrile (3PN), an isomerization step from 2M3BM (not desired) to 3PN and a second hydrocyanation (aided by a Lewis acid
cocatalyst such as aluminium trichloride or triphenylboron) to adiponitrile
.
Naproxen
, an anti-inflammatory
drug, utilizes an asymmetric enantioselective hydrocyanation of vinylnaphthalene
from a phosphinite
(OPR2) ligand, L .The enantioselectivity of this reaction is important because only the S enantiomer
is medicinally desirable, whereas the R enantiomer produces harmful health effects. This reaction can produce the S enantiomer with > 90% selectivity
. Upon recrystallization
of the crude product, the optically pure nitrile can be attained.
the hydrocyanation of linear alkenes.
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and –CN
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s are added to a molecular
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
substrate
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
. Usually the substrate is an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and the product is a nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
. When –CN is a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in a transition metal complex, its basicity makes it difficult to dislodge, so, in this respect, hydrocyanation is remarkable. Since cyanide is both a good σ–donor and π–acceptor its presence accelerates the rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of substitution
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
of ligands trans from itself, the trans effect
Trans effect
In inorganic chemistry, the trans effect is the labilization of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands...
.1 A key step in hydrocyanation is the oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
of hydrogen cyanide to low–valent metal complexes. In hydrocyanation of unsaturated carbonyls addition over the alkene competes with addition over the carbonyl group.
Inorganic Chemistry
Hydrocyanation is performed on alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s with copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
, and most commonly, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
catalysts. Industrial hydrocyanation utilizes phosphite
Phosphite
A phosphite is a salt of phosphorous acid. The phosphite ion is a polyatomic ion with a phosphorus central atom where phosphorus has an oxidation state of +3...
(P(OR)3) complexes of nickel. Phosphites give excellent catalysts, whereas the related phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
(PR3) ligands, which are more basic, are catalytically inactive. Chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
, chelating aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
diphosphite complexes are commonly employed in asymmetric
Symmetry
Symmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
hydrocyanation. An example of a nickel–phosphite catalyzed hydrocyanation of ethene.
Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s, such as B(C6H5)3, can increase hydrocyanation rates and allow for lower operating temperature
Operating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
s. Triphenylboron may derive this ability from sterically protecting the –CN as it is bound to nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. Rates can also be amplified with electron–withdrawing groups (NO2, CF3, CN, C(=O)OR, C(=O)R) on the phosphite ligands, because they stabilize Ni(0)
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
.
A major problem when using nickel catalysts for hydrocyanation is the production of Ni0(CN)x as a result of excess HCN.3 Bulky ligands impede the formation of these unreactive Ni0(CN)x complexes.
Usage
Hydrocyanation is important due to the versatility of alkyl nitriles (RCN), which are important intermediates for the syntheses of amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s, amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s, and ester compounds.
The most popular industrial usage of nickel-catalyzed hydrocyanation is for adiponitrile
Adiponitrile
Adiponitrile is the organic compound with the formula 42. This dinitrile, a viscous, colourless liquid, is an important precursor to the polymer nylon 66. In 2005, about one billion kilograms were produced annually.-Early routes:...
(NC–(CH2)4–CN) synthesis from 1,3–butadiene (CH2=CH–CH=CH2). Adiponitrile is a precursor
Precursor (chemistry)
In chemistry, a precursor is a compound that participates in the chemical reaction that produces another compound. In biochemistry, the term "precursor" is used more specifically to refer to a chemical compound preceding another in a metabolic pathway....
to hexamethylenediamine
Hexamethylenediamine
Hexamethylenediamine is the organic compound with the formula H2N6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid has a strong amine odor, similar to piperidine...
(H2N–(CH2)6–NH2), which is used for the production of certain kinds of Nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
. The DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
ADN process to give adiponitrile is shown below:
This process consists of three steps: hydrocyanation of butadiene to a mixture of 2-methyl-3-butenenitrile (2M3BM) and 3-pentenenitrile (3PN), an isomerization step from 2M3BM (not desired) to 3PN and a second hydrocyanation (aided by a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
cocatalyst such as aluminium trichloride or triphenylboron) to adiponitrile
Adiponitrile
Adiponitrile is the organic compound with the formula 42. This dinitrile, a viscous, colourless liquid, is an important precursor to the polymer nylon 66. In 2005, about one billion kilograms were produced annually.-Early routes:...
.
Naproxen
Naproxen
Naproxen sodium is a nonsteroidal anti-inflammatory drug commonly used for the reduction of pain, fever, inflammation and stiffness caused by conditions such as:...
, an anti-inflammatory
Anti-inflammatory
Anti-inflammatory refers to the property of a substance or treatment that reduces inflammation. Anti-inflammatory drugs make up about half of analgesics, remedying pain by reducing inflammation as opposed to opioids, which affect the central nervous system....
drug, utilizes an asymmetric enantioselective hydrocyanation of vinylnaphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
from a phosphinite
Phosphinite
Phosphinites are organophosphorus compounds with the formula PR2. They are esters of phosphinous acid.-See also:*Phosphine - PR3*Phosphine oxide - OPR3*Phosphonite - P2R*Phosphite - P3*Phosphinate - OPR2*Phosphonate - OP2R...
(OPR2) ligand, L .The enantioselectivity of this reaction is important because only the S enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
is medicinally desirable, whereas the R enantiomer produces harmful health effects. This reaction can produce the S enantiomer with > 90% selectivity
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one...
. Upon recrystallization
Recrystallization (chemistry)
-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
of the crude product, the optically pure nitrile can be attained.
History
Hydrocyanation was first reported by Arthur and Pratt in 1954, when they homogeneously catalyzedHomogeneous catalysis
In chemistry, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent with the reactants.-Acid catalysis:...
the hydrocyanation of linear alkenes.