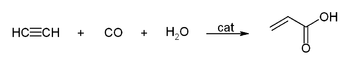
Walter Reppe
Encyclopedia
Walter Julius Reppe was a German chemist
. He is notable for his contributions to the chemistry of acetylene
.
in Munich
in 1920.
In 1921, Reppe worked for BASF
's main laboratory. From 1923, he worked on the catalytic
dehydration of formamide
to prussic acid in the indigo
laboratory, developing this procedure for industrial use. In 1924, he left research for 10 years, only resuming it in 1934.
in 1928. Acetylene is a gas which can take part in many chemical reaction
s. However, it is explosive and accidents often occurred. Because of this danger, small quantities of acetylene were used at a time, and always without high pressures. In fact, it was forbidden to compress acetylene over 1.5 bar at BASF.
Reactions at such low pressures did not correspond at all to the traditions at BASF, and one could not expect any useful process engineering results. Reppe commented in 1949 retrospectively: "therefore the necessity resulted to break with all delivered opinions and to study first of all the acetylene decay with consideration of the most diverse test conditions of reason on, in order to determine suitable safety precautions, one safe working also in the industrial yardstick made possible." To work with acetylene safely, Reppe designed special test tubes, the so-called "Reppe glasses" — stainless steel spheres with screw-type cap, which permitted high pressure experiments. The efforts ended finally with a large number of interconnected reactions, known as Reppe chemistry.
s are called Reppe Chemistry. Reactions can be classified into four large classes:
This simple synthesis was used to prepare acrylic acid
derivatives for the production of acrylic glass
.
If a competing ligand such as triphenylphosphine
is present in sufficient proportion to occupy one coordination site, then room is left for only three acetylene molecules, and these come together to form Benzene
This reaction provided an unusual route to benzene
and especially to cyclooctatetraene
, which was difficult to prepare otherwise.
Products from these four reaction types proved to be versatile intermediates in the syntheses of lacquers, adhesives, foam materials, textile fibers, and pharmaceuticals could now be produced.
from 1951 and 1952 respectively. Together with Otto Bayer
and Karl Ziegler
he received the Werner-von-Siemens-Ring in 1960 for expanding the scientific knowledge on and for the technical development of new synthetic high-molecular materials.
, Karl Ziegler
, Hans Tropsch
, and Franz Fischer
, Reppe was a leader in demonstrating the utility of metal-catalyzed reactions in large scale synthesis of organic compounds. The economic benefits demonstrated by this research motivated the eventual flowering of organometallic chemistry
and its close connection to industry.
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
. He is notable for his contributions to the chemistry of acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
.
Education and career
Walter Reppe began his study of the natural sciences University of Jena in 1911. Interrupted by the First World War, he obtained his doctorateDoctorate
A doctorate is an academic degree or professional degree that in most countries refers to a class of degrees which qualify the holder to teach in a specific field, A doctorate is an academic degree or professional degree that in most countries refers to a class of degrees which qualify the holder...
in Munich
Munich
Munich The city's motto is "" . Before 2006, it was "Weltstadt mit Herz" . Its native name, , is derived from the Old High German Munichen, meaning "by the monks' place". The city's name derives from the monks of the Benedictine order who founded the city; hence the monk depicted on the city's coat...
in 1920.
In 1921, Reppe worked for BASF
BASF
BASF SE is the largest chemical company in the world and is headquartered in Germany. BASF originally stood for Badische Anilin- und Soda-Fabrik . Today, the four letters are a registered trademark and the company is listed on the Frankfurt Stock Exchange, London Stock Exchange, and Zurich Stock...
's main laboratory. From 1923, he worked on the catalytic
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
dehydration of formamide
Formamide
Formamide, also known as methanamide, is an amide derived from formic acid. It is a clear liquid which is miscible with water and has an ammonia-like odor. It is used primarily for manufacturing sulfa drugs and synthesizing vitamins and as a softener for paper and fiber...
to prussic acid in the indigo
Indigo
Indigo is a color named after the purple dye derived from the plant Indigofera tinctoria and related species. The color is placed on the electromagnetic spectrum between about 420 and 450 nm in wavelength, placing it between blue and violet...
laboratory, developing this procedure for industrial use. In 1924, he left research for 10 years, only resuming it in 1934.
Acetylene chemistry
Reppe began his interest in acetyleneAcetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
in 1928. Acetylene is a gas which can take part in many chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s. However, it is explosive and accidents often occurred. Because of this danger, small quantities of acetylene were used at a time, and always without high pressures. In fact, it was forbidden to compress acetylene over 1.5 bar at BASF.
Reactions at such low pressures did not correspond at all to the traditions at BASF, and one could not expect any useful process engineering results. Reppe commented in 1949 retrospectively: "therefore the necessity resulted to break with all delivered opinions and to study first of all the acetylene decay with consideration of the most diverse test conditions of reason on, in order to determine suitable safety precautions, one safe working also in the industrial yardstick made possible." To work with acetylene safely, Reppe designed special test tubes, the so-called "Reppe glasses" — stainless steel spheres with screw-type cap, which permitted high pressure experiments. The efforts ended finally with a large number of interconnected reactions, known as Reppe chemistry.
Reppe chemistry
The high pressure reactions catalysed by heavy metal acetylides, especially copper acetylide, or metal carbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
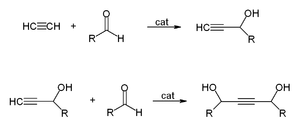
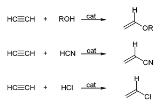
s are called Reppe Chemistry. Reactions can be classified into four large classes:
- The vinylVinylA vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
ization according to the equation:
- Preparing ethynyldiols from aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s according to the equation:
- Reactions with carbon monoxideCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
:
This simple synthesis was used to prepare acrylic acid
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
derivatives for the production of acrylic glass
Acrylic glass
Poly is a transparent thermoplastic, often used as a light or shatter-resistant alternative to glass. It is sometimes called acrylic glass. Chemically, it is the synthetic polymer of methyl methacrylate...
.
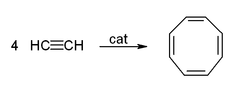
- The cyclic polymerizationPolymerizationIn polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
or cyclo-oligomerizationOligomerIn chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
of acetylene to cyclooctatetraeneCyclooctatetraene1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
, which is one of the most important applications of template reactions. The reaction occurs at a nickelNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
(II) centre, where it is supposed that four acetylene molecules occupy four sites around the metal, and react simultaneously to give the product.
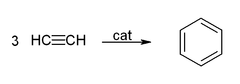
If a competing ligand such as triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
is present in sufficient proportion to occupy one coordination site, then room is left for only three acetylene molecules, and these come together to form Benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
This reaction provided an unusual route to benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and especially to cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
, which was difficult to prepare otherwise.
Products from these four reaction types proved to be versatile intermediates in the syntheses of lacquers, adhesives, foam materials, textile fibers, and pharmaceuticals could now be produced.
Post-war
After the Second World War, Reppe led the research of BASF from 1949 up to his retirement in 1957. From 1952 to 1966, he also sat on the supervisory board. He was also a professor at the University of Mainz and TH DarmstadtDarmstadt
Darmstadt is a city in the Bundesland of Hesse in Germany, located in the southern part of the Rhine Main Area.The sandy soils in the Darmstadt area, ill-suited for agriculture in times before industrial fertilisation, prevented any larger settlement from developing, until the city became the seat...
from 1951 and 1952 respectively. Together with Otto Bayer
Otto Bayer
Otto Bayer was a German industrial chemist at IG Farben who was head of the research group that discovered the polyaddition for the synthesis of polyurethanes out of polyisocyanate and polyol. It may be noted that although Dr. Bayer shared the name of his venerable employer, he was not actually...
and Karl Ziegler
Karl Ziegler
Karl Waldemar Ziegler was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compounds [which]...led to new polymerization reactions and ... paved the way for new and highly...
he received the Werner-von-Siemens-Ring in 1960 for expanding the scientific knowledge on and for the technical development of new synthetic high-molecular materials.
Legacy
Most of the industrial processes that were developed by Reppe and coworkers have been superseded, largely because acetylenes are expensive, high energy species relative to alkenes which are more cheaply produced. Together with his contemporaries Otto RoelenOtto Roelen
Otto Roelen was a German chemist.Roelen studied chemistry and graduated in 1922 from Technische Hochschule Stuttgart. He worked with Franz Fischer and Hans Tropsch at the Kaiser Wilhelm Institute for Coal Research from 1922...
, Karl Ziegler
Karl Ziegler
Karl Waldemar Ziegler was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compounds [which]...led to new polymerization reactions and ... paved the way for new and highly...
, Hans Tropsch
Hans Tropsch
Hans Tropsch was a chemist responsible, along with Franz Fischer, for the development of the Fischer-Tropsch process.- Life :...
, and Franz Fischer
Franz Joseph Emil Fischer
Franz Joseph Emil Fischer was a German chemist. He and Hans Tropsch discovered the Fischer-Tropsch process. With Hans Schrader he developed the Fischer Assay, a standardized laboratory test for determining the oil yield from oil shale to be expected from a conventional shale oil extraction...
, Reppe was a leader in demonstrating the utility of metal-catalyzed reactions in large scale synthesis of organic compounds. The economic benefits demonstrated by this research motivated the eventual flowering of organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
and its close connection to industry.
Publications
- Neue Entwicklungen auf dem Gebiet der Chemie des Acetylen und Kohlenoxyds (New developments in the area of the chemistry acetylene and carbon monoxide). Springer Berlin, Göttingen, Heidelberg. 1949. 184 pages.