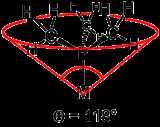
Ligand cone angle
Encyclopedia
The ligand cone angle is a measure of the size of a ligand
. It is defined as the solid angle
formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone (see figure). Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was introduced by Chadwick A. Tolman
, a research chemist at Dupont. Originally applied to phosphines, these cone angles were originally determined by building scale models of these phosphines, and physically measuring the angle of the cone by a home-made device.
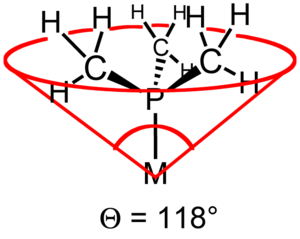
assuming a bite angle of 74, 85, 90 deg. for diphosphines with methylene, ethylene, and propylene back bones respectively.

because the size of the ligand affects the reactivity of the attached metal center. In a famous example, the selectivity of hydroformylation
catalysts is strongly influenced by the size of the coligands.
One remarkable feature becomes clear from these data: some ligands occupy more than half of the coordination sphere
of a metal center.
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. It is defined as the solid angle
Solid angle
The solid angle, Ω, is the two-dimensional angle in three-dimensional space that an object subtends at a point. It is a measure of how large that object appears to an observer looking from that point...
formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone (see figure). Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was introduced by Chadwick A. Tolman
Chadwick A. Tolman
Chadwick A. Tolman is an American chemist. He obtained his B.S. in Chemistry from Massachusetts Institute of Technology. He earned his Ph.D. in Chemistry as a microwave spectroscopist from U.C. Berkeley under the guidance of Professor William Gwinn.He joined DuPont Central Research in 1965. ...
, a research chemist at Dupont. Originally applied to phosphines, these cone angles were originally determined by building scale models of these phosphines, and physically measuring the angle of the cone by a home-made device.

Asymmetric cases
The concept of cone angle is most easily visualized with symmetrical ligands, e.g. PR3. But the approach has been refined to include less symmetrical ligands of the type PRR'R" as well as diphosphines. In such asymmetric cases the substituent angles half angles, θi/2, are averaged and then doubled to find the total cone angle, θ. In the case of diphosphines the θi/2 of the backbone is approximated as half the chelate bite angleBite angle
The bite angle is a geometric parameter used to classify chelating ligands in inorganic and organometallic chemistry. Together with ligand cone angle, this parameter is relevant to diphosphine ligands, which are used in industrial processes such as hydroformylation and hydrocyanation...
assuming a bite angle of 74, 85, 90 deg. for diphosphines with methylene, ethylene, and propylene back bones respectively.

Variations
The Tolman cone angles assume empirical bond data and defines the perimeter as the maximum possible circumscription of an idealized free spinning substituent. In contrast the solid-angle concept derives both bond length and the perimeter form empirical solid state crystal structures. There are advantages to each system.Application
The concept of cone angle is of practical importance in homogeneous catalysisHomogeneous catalysis
In chemistry, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent with the reactants.-Acid catalysis:...
because the size of the ligand affects the reactivity of the attached metal center. In a famous example, the selectivity of hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
catalysts is strongly influenced by the size of the coligands.
Cone angle values
Cone angles of common phosphine ligands in degrees:| Ligand | Angle (°) |
|---|---|
| PH3 Phosphine Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine... |
87 |
| PF3 Phosphorus trifluoride Phosphorus trifluoride , is a colorless and odorless gas. It is highly toxic and it reacts slowly with water. Its main use is as a ligand in metal complexes... |
104 |
| P(OCH3)3 | 107 |
| dmpe 1,2-Bis(dimethylphosphino)ethane 1,2-Bisethane is a diphosphine ligand in coordination chemistry. It can be synthesised by the reaction of methylmagnesium iodide with 1,2-bisethane:... |
107 |
| depe | 115 |
| P(CH3)3 Trimethylphosphine Trimethylphosphine is the organophosphorus compound with the formula P3, commonly abbreviated PMe3. This colorless liquid has a strongly unpleasant odour, which is characteristic of alkylphosphines. It is a pyramidal molecule with C3v symmetry, similar to ammonia and phosphine . As a ligand, its... |
118 |
| dppm 1,1-Bis(diphenylphosphino)methane 1,1-Bismethane , is an organophosphorus compound with the formula CH22. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand... |
121 |
| dppe 1,2-Bis(diphenylphosphino)ethane 1,2-Bisethane is a commonly used bidentate ligand in coordination chemistry. Dppe is almost invariably chelated, although there are examples of unidentate and of bridging behavior.-Preparation:... |
125 |
| dppp 1,3-Bis(diphenylphosphino)propane 1,3-Bispropane is a diphosphine often found as a ligand in coordination chemistry.The diphosphine can be prepared by the reaction of lithium diphenylphosphide and 1,3-dichloropropane.The diphosphine is a precursor to the complex dichloronickel, which is prepared by combining equimolar portions of... |
127 |
| P(CH2CH3)3 | 132 |
| dcpe | 142 |
| P(C6H5)3 Triphenylphosphine Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature... |
145 |
| P(cyclo-C6H11)3 Tricyclohexylphosphine Tricyclohexylphosphine is the tertiary phosphine with the formula P3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to PCy3, where Cy stands for cyclohexyl... |
170 |
| P(t-Bu)3 | 182 |
| P(C6F5)3 | 184 |
| P(2,4,6-Me3C6H2)3 | 212 |
One remarkable feature becomes clear from these data: some ligands occupy more than half of the coordination sphere
Coordination sphere
In coordination chemistry, the coordination sphere refers to a central atom or ion and an array of molecules or anions, the ligands, around.Molecules that are attached noncovalently to the ligands are called the second coordination sphere....
of a metal center.
See also
- Bite angleBite angleThe bite angle is a geometric parameter used to classify chelating ligands in inorganic and organometallic chemistry. Together with ligand cone angle, this parameter is relevant to diphosphine ligands, which are used in industrial processes such as hydroformylation and hydrocyanation...
- Steric effectsSteric effectsSteric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
(vs. electronic effects) - Tolman electronic parameterTolman electronic parameterThe Tolman electronic parameter , named after Chadwick A. Tolman, is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode of a complex, LNi3 by infrared spectroscopy, where L is the ligand being studied...

